Hey everyone, this is just an extra part of the previous tutorial in which we covered the mole and how we can calculate the mass of a sample of a substance using the amount in moles and vice-versa.
In this extra portion, I’m going to run through some more calculations, only this time we are only going to use molar mass as a variable and introduce an additional step involving conversion factors.
Note: The use of conversion factors in mole and mass calculations is required in some exams – however the requirements of different exams vary.
I would advise you seek advice from your teacher, lecturer or tutor on whether to use conversion factors in your exams. If you don’t need to use conversion factors in mole and mass calculations in your exams, please refer to the methods and calculations shown in ‘Moles, Mass and Avogadro’s Constant’ in which no conversion factors are used.
However, if you need to use conversion factors in your calculations then please read on.
What is a conversion factor?
A conversion factor is a fraction that consists of one value with a particular unit and an equivalent value featuring a different unit. The main purpose of a conversion factor is to allow for the conversion between a measurement featuring one unit to a measurement featuring a different unit. This is achieved in a calculation by cancelling out the unit we want to convert from, thereby only leaving the unit we want to convert to for our answer. Let’s see how this happens with an example that doesn’t feature the mass or moles of a chemical substance first.

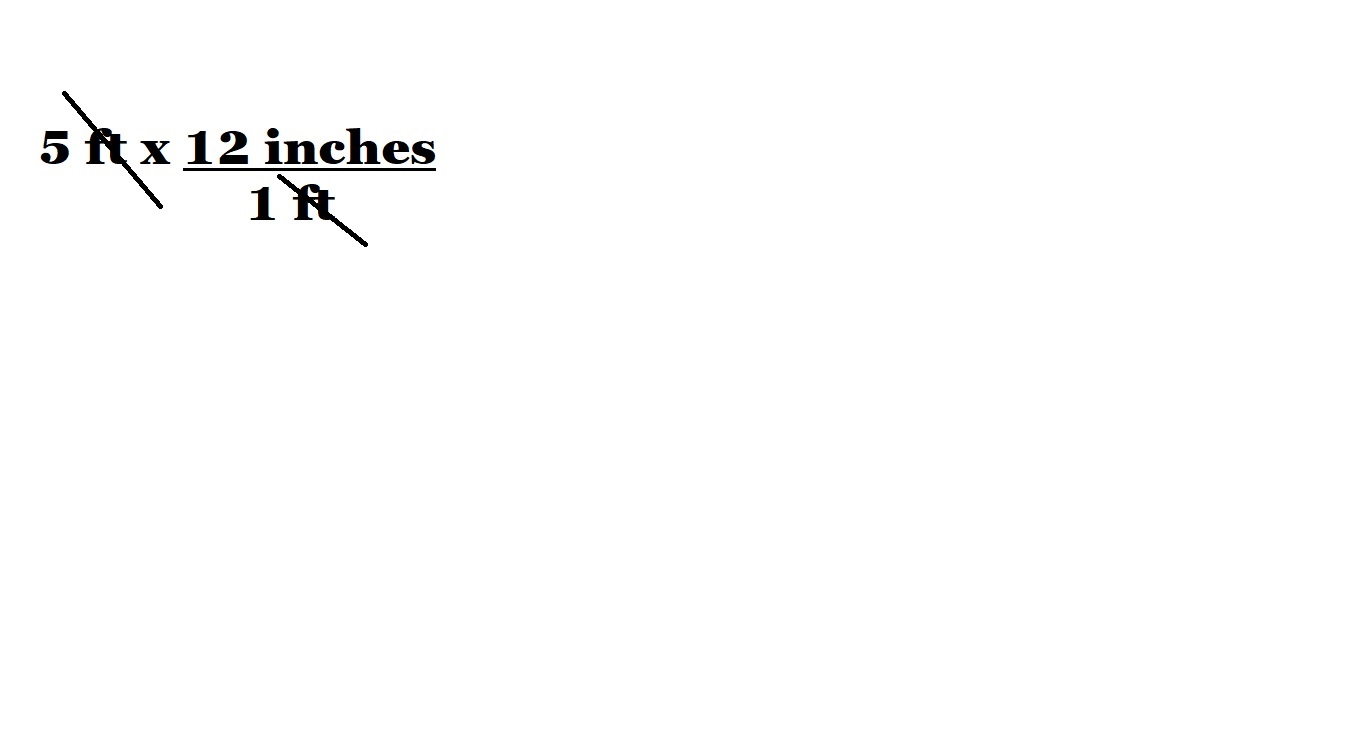
In the example above, we are trying to convert from a measurement in feet to a measurement in inches.
To convert from a measurement in feet to a measurement in inches, we want to cancel out the unit of feet from this calculation. To do this, we have included a conversion factor featuring 1 foot (1 ft) as the denominator. 1 foot is the equivalent to 12 inches because there are 12 inches in one foot.
The inclusion of this conversion factor means we now have the unit we want to cancel out at the top of our fraction (the numerator) and the bottom of our fraction (the denominator). This now means we can can cancel out the unit of feet as follows:
This then allows us to carry out the calculation but only with the unit that we want for our answer – which in this calculation is inches.

As you can see, dividing by 1 doesn’t change the numerical value of the answer, it was just used as a way of implementing a conversion factor to cancel out the unit we didn’t want for our answer.
Okay let’s carry out a calculation in which we use the mass of a substance to work out the amount of a substance in moles using molar mass and a conversion factor.
Reminder: All the calculations featured will involve the use of the variable molar mass. The use of conversion factors do not apply to calculations involving relative formula mass because relative formula mass does not have units.
Note: You will need a periodic table for these calculations.
Calculating Moles using Mass, Molar Mass and a Conversion Factor
Question: How many moles of molecules are there in 6.50 g of sulphur dioxide (SO2)?
Step 1: Use the correct formula.
The formula we are going to use for this calculation is the same as the one we used for working out the number of moles present using mass and molar mass without a conversion factor. That formula is the following:

Step 2: Work out the molar mass of the substance involved in the calculation.
We work out the molar mass in exactly the same way we did when we didn’t use a conversion factor – which is by adding up the relative atomic masses of atoms of the elements present in the substance.
So for this question we add the relative atomic mass of 1 sulphur atom to the relative atomic mass of an oxygen atom multiplied by 2 because there are 2 oxygen atoms in the sulphur dioxide compound. We can write this out as follows:
M = 32.1 + (2) (16.0) = 64.1 g mol-1
Step 3 : Include a conversion factor and cancel out units. (This is where things are a little bit different.)
Now we know the molar mass of sulphur dioxide we know the relevant values of the variables in the formula.
However, if we want to cancel out the unit of grams (g) because that is the unit we are converting from, we need to include a conversion factor.

The calculation above now contains a conversion factor and there are a couple of things to note.
Firstly, because we are including a conversion factor, we include the chemical formula of the substance in the calculation in the units of molar mass rather than mol-1. So in this example the molar mass is expressed as 64.1 g SO2.
Secondly, the conversion factor contains 1 mol SO2 because this is the equivalent of the molar mass of sulphur dioxide because the molar mass is the mass of 1 mole.
Thirdly, we now have the unit of grams both at the top of the fraction in the calculation (the numerator) and the bottom of the fraction (the denominator), which means we can now cancel out the unit of grams – as shown below:

Step 4: Carry out the calculation
We can now carry out the calculation involving only the unit we want in our answer – which is moles (mol).
We should make sure that our answer is rounded to the same number of significant figures that are in the value of the variable with the fewest significant figures in the question. In this calculation that would be 3 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.

Multiplying by the 1 in the conversion factor does not change the numerical value of the answer: it only cancels out the units we don’t want for our answer.
So we have worked out that there are 0.101 moles (rounded to 3 significant figures) of sulphur dioxide molecules in 6.50 g of sulphur dioxide.
Let’s now try a calculation in which we work out the mass of a sample of a substance using the known amount in moles, the molar mass and a conversion factor.
Calculating Mass using Moles, Molar Mass and a Conversion Factor
Question: What is the mass of 4.00 moles of sodium chloride (NaCl)?
Step 1: Use the correct formula.
The formula we are going to use for this calculation is the same one we used in the previous tutorial in which mass is the subject and moles and molar mass are the variables involved in the calculation.
That formula is the following:

Step 2: Work out the molar mass of the substance involved in the calculation
This time we have to work out the molar mass of sodium chloride. Don’t forget that despite the fact we’re dealing with an ionic compound consisting of ions, we still have to consider the relative atomic masses of atoms of the elements present in the compound.
So for this calculation we are adding the relative atomic mass of 1 sodium atom to the relative atomic mass of 1 chlorine atom. We can write this out as follows:
M = 23.0 + 35.5 = 58.5 g mol-1
Step 3: Include a conversion factor and cancel out units
This time the unit we want to convert from and therefore the unit we want to cancel out is moles (mol). To do this we must write out the following:

The conversion factor in the calculation contains 1 mol NaCl. This is because 1 mole of sodium chloride is the equivalent to the molar mass of sodium chloride because the molar mass is the mass of 1 mole.
However, whereas in the last calculation 1 mole of the substance involved was part of the numerator (at the top) of the fraction, this time it’s part of the denominator (at the bottom of the fraction) in the calculation.
The unit of moles is at the top of the fraction because of the way in which the formula is organised; therefore in order to cancel out the unit of moles, we must have the unit of moles at the bottom of the conversion factor.
Note once again how the unit of mol-1 in molar mass is replaced by the chemical formula of NaCl.
Now we can cancel out the unit of moles which leaves only the unit of grams (g) – which is the unit we want for our answer.

Step 4: Carry out the calculation.
We can now carry out the calculation with only the unit we want for our answer – which is grams (g).
This time we should round our answer to 3 significant figures because the variable with the fewest significant figures consists of 3 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.

So we have worked out that the mass of a sample containing 4.00 moles of sodium chloride is 234 g.
Finished
So there we go, now we know how to use conversion factors to cancel out the units in the measurement we want to convert from when changing between measurements of mass in grams, to amount in moles and vice-versa.
I would like to state once again that this additional step may be required in some exams in order to earn a few extra marks – however the requirements for different exams vary.
I would advise once again that you ask your teacher, lecturer or tutor whether you need to use conversion factors in your calculations involving molar mass or whether you should use the method we went over in the last tutorial – where conversion factors were not used – for your exams.
I’ll also be uploading a video version of this extra piece of this tutorial on my YouTube channel soon.
In the next tutorial, I’ll be going over how to convert between a measurement of the amount of a substance in particles to moles and vice-versa using Avogadro’s number. channel soon.
Thanks for reading and goodbye for now.