In the last tutorial, we explored how the mole is used as a unit of measurement of amount in chemistry and how we calculate the amount of a substance in moles. We discussed how a particular number known as Avogadro’s number is involved with the mole. We also covered how the mole relates to mass and how the mole can be used to calculate the mass of a substance.
In this tutorial, we are going to see how Avogadro’s number can be used once again as an effective tool; this time we’ll see how it can be used to convert between a measurement of the amount of a substance in particles – including atoms, molecules and formula units – to a measurement of amount in moles and vice-versa.
Okay, let’s have a quick recap on what Avogadro’s number is. Avogadro’s number is the number of particles that are present in one mole of a substance. This number is given as a value (in standard form or scientific notation) of 6.02 x 1023 mol-1 to three significant figures.
Reminder: In standard form/scientific notation, only the figures in the multiplier (the number before the multiplication sign) are significant figures.
Reminder: It is common practice in chemistry and the other sciences to round numbers to a certain number of significant figures – which is often the same number of significant figures that are present in the value with the fewest significant figures in the question.
In this tutorial we will be considering the value of Avogadro’s number in three significant figures – however the value can be expressed with more significant figures (such as 6.022 x 1023 mol-1 which is the value of Avogadro’s number in four significant figures).
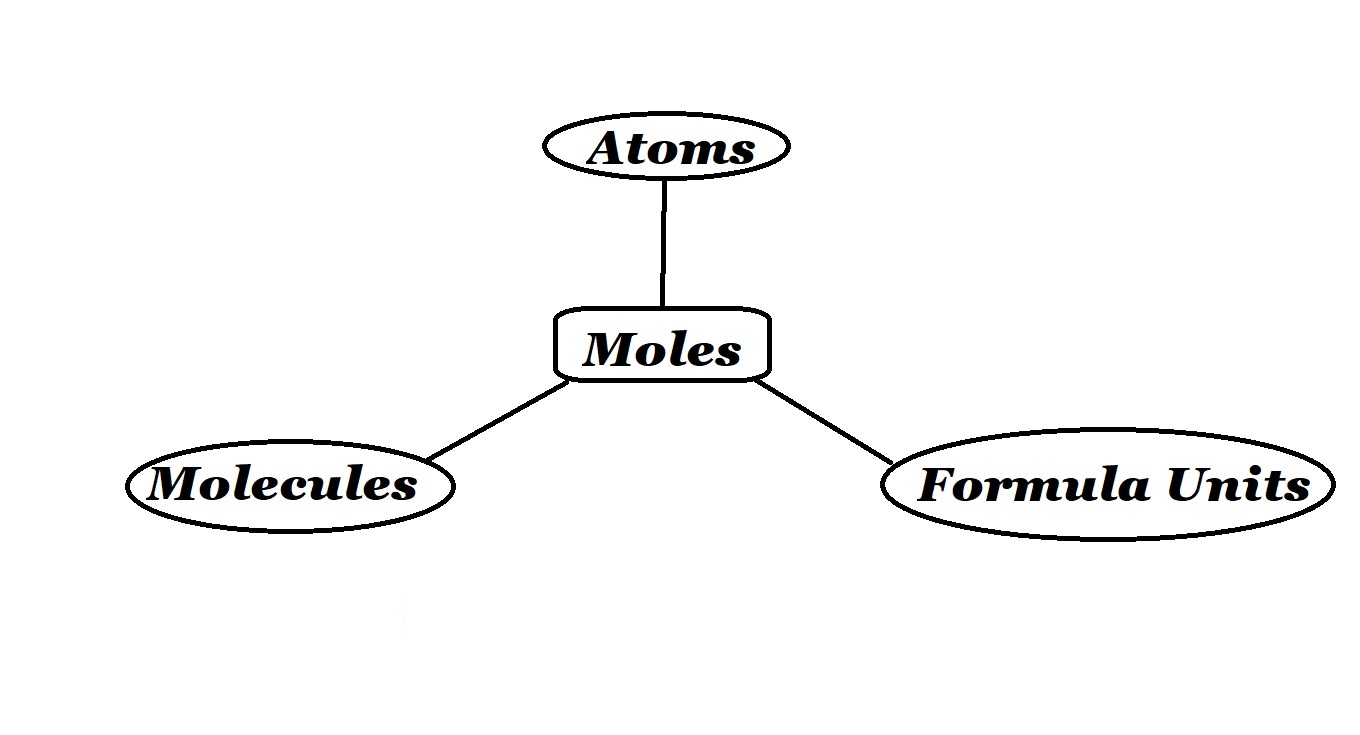
Let’s also quickly remind ourselves that when we say ‘amount’ in chemistry, we are talking about the number of particles of a substance that are present in a sample.
Okay let’s now see how Avogadro’s number (of the principle Avogadro’s Constant) can be used to convert from a measurement of amount expressed in atoms, molecules or formula units to a measurement of amount expressed in moles.
Mathematical Formulas for Conversions
This process – like a lot of processes in science – involves the use of a formula. The variables present in the formula depend on whether you are converting atoms, molecules or formula units to moles.

According to the formulas above, in order to calculate the amount of a substance in moles when you know the number of atoms, molecules or formula units present, you have to divide the value of the number of particles present by the value of Avogadro’s number – which is 6.02 x 1023 .
Okay, lets look at some calculations.
Calculation 1 : Atoms to Moles
Question: How many moles consists of 3.00 x 1011 sulphur atoms?
Step 1: Use the correct formula.
The first thing we should do is put the known values into our formula.

Reminder: We write the chemical formula of the substance involved for each variable in a calculation that involves using a conversion factor.
Step 2: Include a conversion factor and cancel out units
Now the next thing we have to do is cancel out the units we don’t want for our answer. These are the units that are used to express the measurement of amount we want to convert from. In this example the unit we want to cancel out is atoms. To do this we need to use a conversion factor.
Reminder: A conversion factor is a fraction featuring a value with a particular unit and an equivalent value featuring a different unit. The main purpose of a conversion factor is to allow for the conversion between a measurement featuring one unit to a measurement featuring a different unit. This is achieved in a calculation by cancelling out the unit we want to convert from, thereby only leaving the unit we want to convert to for our answer.
Let’s include the conversion factor we need for this calculation and see how it allows us to cancel out the unit we don’t need.

Reminder: We use brackets to separate values which are going to be multiplied with one another. This is to avoid the confusing situation where we would have multiplication signs in standard form values and in between values of variables that are going to be multiplied.
The conversion factor for this conversion features 1 mol S because 1 mole of sulphur atoms is the equivalent to 6.02 x 1023 sulphur atoms because there are 6.02 x 1023 sulphur atoms in 1 mole.
However, in order for the conversion factor to work you must write 1 mol – followed by the chemical formula of the substance involved – as part of the numerator. Avogadro’s number must be written numerically in standard form/scientific notation as the denominator to convert from particles to moles.
So let’s see how it cancels out the unit of atoms in the calculation.

To cancel out the unit of atoms, we must multiply the value of sulphur atoms in the sample by the conversion factor. This means that we have the unit of atoms in the numerator and the denominator of the fraction, which means we can cancel out the unit of atoms.
Step 3: Carry out the calculation
Okay, so now we have cancelled out the unit we don’t need, we can carry out the calculation. Now this can be done by typing in the following into a calculator and will result in the following answer.

Note that multiplying by 1 doesn’t change the answer numerically – it only allowed us to include a conversion factor to cancel out the unit we didn’t want for our answer.
Don’t forget to round your answer to the same number of significant figures that are present in the value with the fewest significant figures in the question. So we round our answer to the above calculation to three significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.
So we have worked out that 3.00 x 1011 sulphur atoms are within 4.98 x 10 – 13 moles of sulphur.
Let’s try another calculation.
Calculation 2: Molecules to Moles
Question: How many moles consists of 7.0 x 10 9 nitrogen dioxide (NO2) molecules?
Step 1: Use the correct formula
Okay we start just like we did previously by putting in the values we know into the correct formula.

Step 2: Include a conversion factor and cancel out units
Then we cancel out the unit we don’t want for our answer, which this time is molecules so we should include a suitable conversion factor.

The conversion factor features 1 mol NO2 because this is the equivalent of 6.02 x 1023 molecules of nitrogen dioxide in moles.
We can now cancel out the molecules unit because it’s part of the numerator and the denominator.

We are then left with the following:

Step 3: Carry out the calculation
Okay let’s now carry out the calculation:

In this calculation we have to round our answer to 2 significant figures because the value with the fewest significant figures in the question consists of 2 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in two significant figures rather than one significant figure.
So we have worked out (to two significant figures) that 1.2 x 10 -14 moles of nitrogen dioxide consists of 7.0 x 109 molecules of nitrogen dioxide.
Calculation 3: Formula Units to Moles
Question: How many moles consists of 6.28 x 1014 formula units of magnesium oxide (MgO)?
Step 1: Use the correct formula

The unit we are converting from and therefore the unit we want to cancel out this time is formula units, so we should include a conversion factor that contains the equivalent value of 6.02 x 1023 formula units of MgO. This is 1 mol MgO because there are 6.02 x 1023 formula units of magnesium oxide in one mole of magnesium oxide.

So now that we have the unit of formula units in the numerator and denominator, we can now cancel out the unit of formula units.

We are then left with the following:

Step 3: Carry out the calculation

We round our answer to three significant figures because the value with the fewest significant figures in the question consists of 3 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.
So we have worked out that 1.04 x 10 – 9 moles of magnesium oxide consists of 6.28 x 1014 magnesium oxide formula units.
Okay so now we know how to convert from a measurement of the amount of a substance in particles to moles. Let’s now look at how we can convert from a measurement in moles to a measurement in particles.
Amount in Moles to Amount in Particles
To convert from moles to atoms, molecules or formula units, we need to rearrange the formula we used before. We need to rearrange the formula so amount measured in number of atoms, molecules or formula units is the subject of the formula and as a result we get three possible formulas that look like this:

According to the formulas, in order to work out the number of atoms, molecules or formula units of a substance in a given sample, we must multiply the amount of the substance in moles by Avogadro’s number.
So let’s do some calculations.
Calculation 1: Moles to Atoms
Question: How many helium atoms are present in 9.50 moles of helium (He)?
Step 1: Use the correct formula.

Step 2: Include a conversion factor and cancel units
Now once again we are going to need a conversion factor because we have a unit that we don’t want for our answer and we need a different unit for our answer. The unit we don’t want this time is moles (mol) and the one we want is atoms.
However, unlike the calculations that involve converting from particles to moles, the conversion factor for converting from moles to particles contains 1 mole of the substance involved in the denominator rather than the numerator.
This is due to the arrangement of the variables in the formula for the calculation as both the amount in moles and Avogadro’s number have to be part of the numerator.
So if we were to include a conversion factor, the calculation would look like this:

1 mole of helium is the equivalent to 6.02 x 1023 atoms of helium because there are 6.02 x 1023 helium atoms in 1 mole of helium.
Now we can cancel out the unit we don’t want, which this time is the mole.

After cancelling out the unit of the mole, we are left with the following:

We can then carry out the calculation and we have to give our answer to 3 significant figures because the value with the fewest significant figures in the question consists of 3 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.

So following our calculation, we have worked out that 5.72 x 1024 helium atoms (to three significant figures) are present in 9.50 moles of helium.
Calculation 2: Moles to Molecules
Let’s try another calculation.
Question: How many molecules of carbon dioxide (CO2) are present in 6.5 moles of carbon dioxide?
Step 1:Use the correct formula
Okay let’s put the values we know into the correct formula.

Step 2: Include a conversion factor and cancel out units.
Let’s now include a conversion factor and cancel out the unit we don’t want and only have the unit we want for our answer.
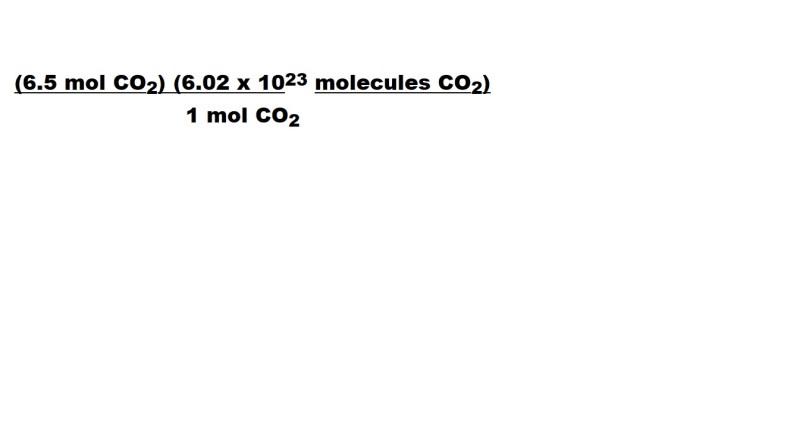
1 mol CO2 is part of the conversion factor because 1 mole CO2 is the equivalent to 6.02 x 1023 molecules CO2 because there are 6.02 x 1023 CO2 molecules in 1 mole of CO2.
Let’s now cancel out the unit we don’t want – which once again is the mole.
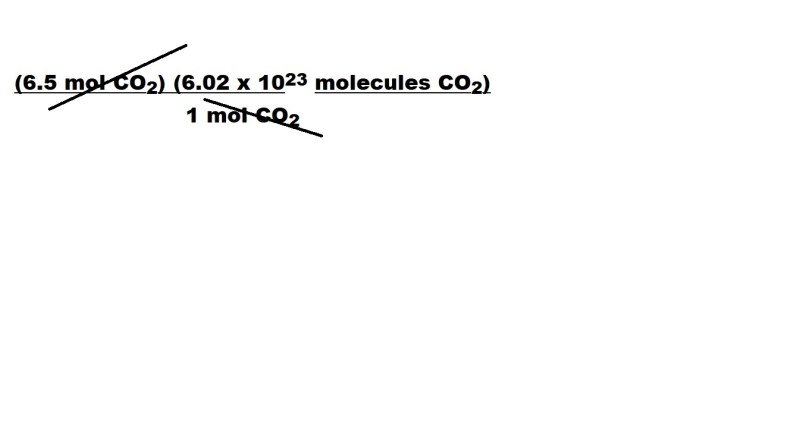
After cancelling out the unit of the mole, we are left with the following:

Step 3: Carry out the calculation
We then carry out the calculation and give our answer to 2 significant figures because the value with the fewest significant figures in the question consists of 2 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in two significant figures rather than one significant figure.

So following our calculation we have worked out that 3.9 x 1024 molecules (to two significant figures) of carbon dioxide molecules are present in 6.5 moles of carbon dioxide.
Calculation 3: Moles to Formula Units
Question: How many formula units of calcium carbonate (CaCO3) are present in 5.70 moles of calcium carbonate (CaCO3)?
Step 1: Use the correct formula
Let’s put the values we have into the correct formula.

Step 2: Include a conversion factor and cancel out units.
Now we have to include a conversion factor to cancel out the unit we don’t want for our answer – which is moles.
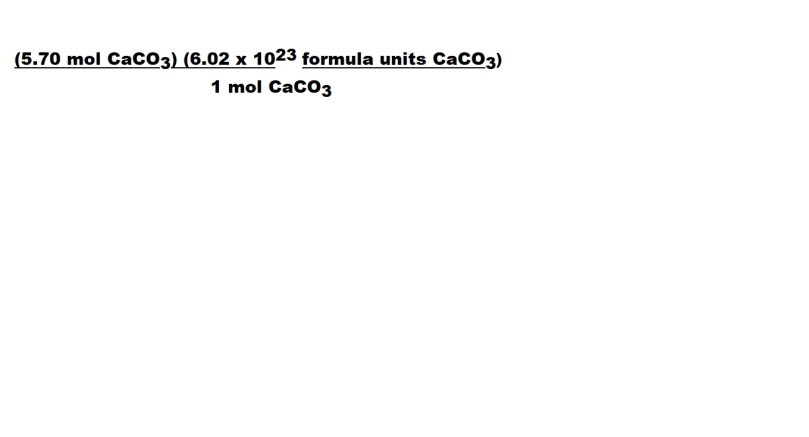
1 mole CaCO3 is part of the conversion factor because 1 mole of CaCO3 is the equivalent to 6.02 x 1023 formula units of CaCO3 .
We can now cancel out the unit of moles because the unit is part of the numerator and the denominator.
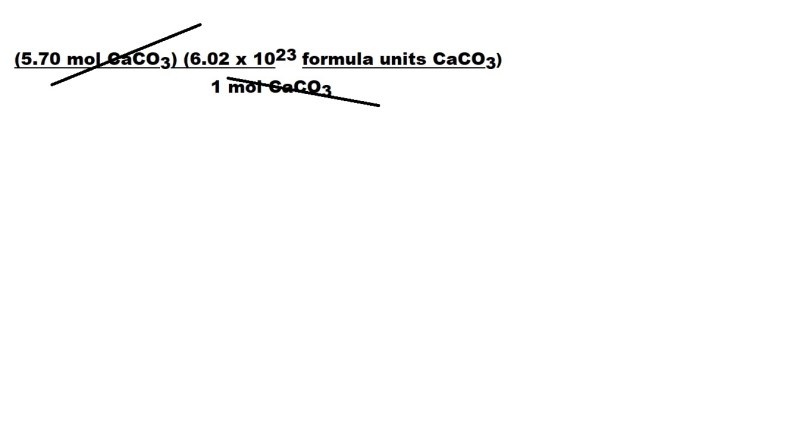
We are then left with the following:

Step 3: Carry out the calculation
We then carry out the calculation and we have to give our answer to 3 significant figures because the value with the fewest significant figures in the question consists of 3 significant figures.
Note: We don’t consider the 1 in the conversion factor when we decide how many significant figures should be in our answer – therefore we express the answer in three significant figures rather than one significant figure.
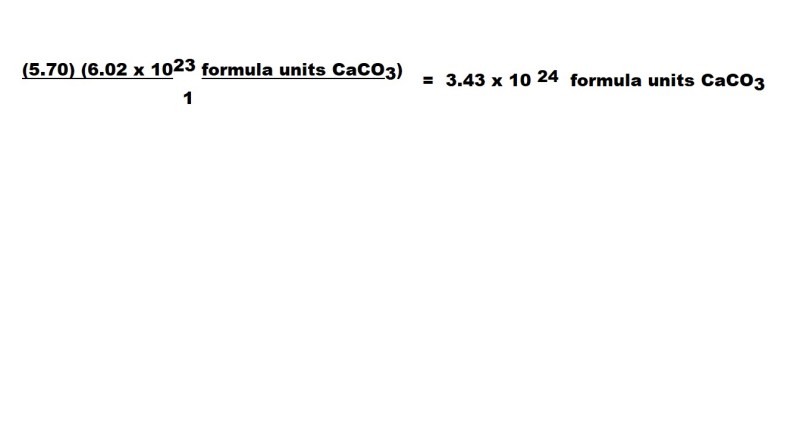
So we have worked out that 3.43 x 1024 formula units of calcium carbonate are present in 5.70 moles of calcium carbonate.
Okay, so now we know how we can use Avogadro’s number to convert between a measurement of the amount of a substance in particles to moles and vice-versa. Once again we have seen how that number plays a crucial role in chemistry.
In the next tutorial, I’m going to be going over how we can calculate the masses of reactants and products of a chemical reaction using a balanced equation. This will involve delving into an area of chemistry known as stoichiometry.
Bye for now.