In this tutorial, we are going to be working out the number of particles – which could be atoms, molecules or formula units – of a substance that are acting as either a reactant or a product of a chemical reaction.
This process involves certain features that we have already used in stoichiometry including: balanced chemical equations; mole ratios and molar masses.
The only difference this time is that we are going to be using Avogadro’s number for the final step in each calculation.
We have met Avogadro’s number in previous tutorials on the mole – but let’s have a quick recap.
6.02 x 10 23
Avogadro’s number (illustrated above) signifies the number of particles that are present in one mole of a substance. The number can be used in stoichiometry to work out the number of particles of a particular substance that is a reactant or a product in a chemical reaction.
Note: We are going to consider Avogadro’s number in three significant figures for these questions. Only the figures in the variable before the multiplication sign (known as the multiplier) are considered significant figures.
The number of particles involved is calculated by multiplying the amount in moles of the chemical substance by Avogadro’s number as illustrated by the formulas below.

This will be the final step in answering the questions in this tutorial.
Okay, so let’s go through some questions.
There are two questions: the first one will involve going from the known amount in moles of a substance to the number of particles of another substance; the second will involve going from the mass of a substance in grams to the number of particles of another substance.
The second question will involve an additional step that won’t be required to answer the first – however you could be asked either one or possibly both types of question in a test or exam.
We will be going through two methods for each question: one involving conversion factors and one without the use of conversion factors.

A summary of the steps involved in answering the questions exemplified in this tutorial.
Question: How many molecules of oxygen react with calcium to produce 5.43 moles of calcium oxide?
Without Conversion Factors
Step 1: Write out and balance the chemical equation that illustrates the reaction.
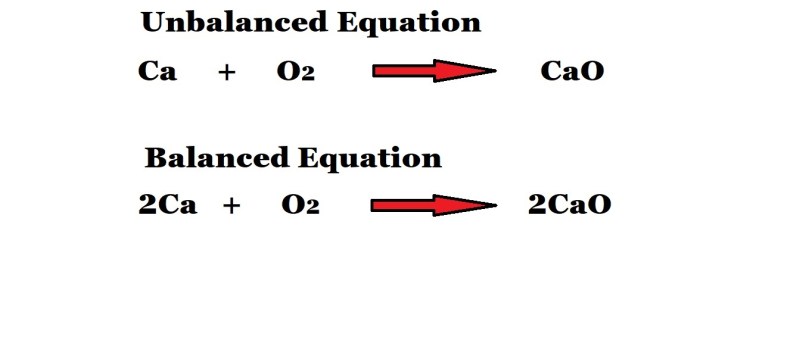
You may be given the equation already balanced in a test or you may be given the unbalanced version to see if you know how stoichiometric coefficients work.
Step 2: Determine the mole ratio (stoichiometric ratio) between oxygen and calcium oxide.
According to the stoichiometric coefficients in the balanced equation, the mole ratio of oxygen to calcium oxide is 1 mole to 2 moles.
Step 3: Use the mole ratio (stoichiometric ratio) to determine the number of moles of oxygen needed as a reactant.
Step 4: Multiply the number of moles of oxygen needed as a reactant by Avogadro’s number in order to determine the number of oxygen molecules needed.
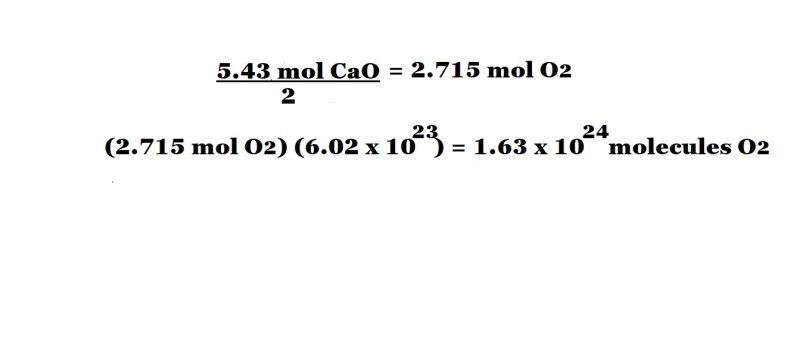
The calculations above show that we need to divide the number of moles of calcium oxide produced by 2 in order to work out the number of moles of oxygen needed as a reactant. This is due to the mole ratio of oxygen to calcium oxide being 1 mole to 2 moles – which means the amount in moles of oxygen would be half the amount in moles of calcium oxide produced.
Please note that we have given the number of moles of oxygen in the reaction in four significant figures; this is because this is not the final answer to the question and we want to avoid rounding to too few significant figures early in the calculation to avoid inaccuracy in our final answer.
We then multiply the number of moles of oxygen participating as a reactant by Avogadro’s number and we get our final answer to the question which we give to three significant figures because the information we were originally given was in three significant figures.
With conversion factors
Steps 1 and 2 are the same as the first two steps in the method that does not involve conversion factors.
Step 3: Organise the calculation to include the given amount in moles of calcium oxide followed by two conversion factors: one that shows the mole ratio between oxygen and calcium oxide and one that consists of Avogadro’s number with the unit of molecules of oxygen and the equivalent value of 1 mole of oxygen.
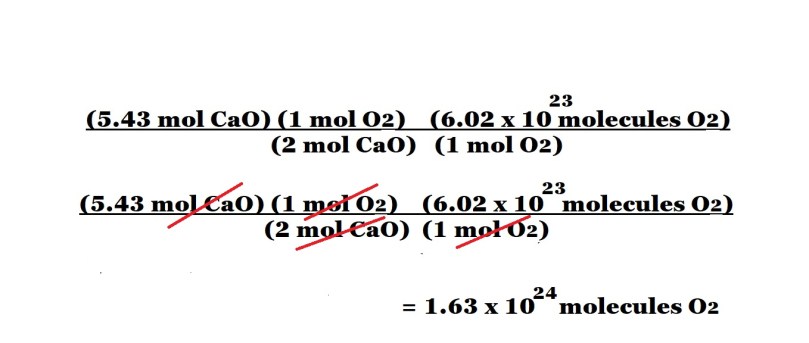
From left to right: The amount in moles of calcium oxide; the number of moles of oxygen according to the mole ratio as the numerator and the number of moles of calcium oxide in the mole ratio as the denominator; Avogadro’s number of molecules of oxygen and the equivalent value of 1 mole of oxygen because Avogadro’s number is the number of particles in 1 mole of a substance.
The placement of the above variables are important because we want to cancel out the units we don’t want for our final answer and only leave the unit we want – which in this question is molecules of oxygen.
Once we have cancelled out the unwanted units, we can carry out the calculation with just numbers except for the unit we want for our answer.
So both of the aforementioned methods led to the same answer.
Let’s now carry out our second question, but this time we only know the mass of a substance in grams and we want to know how many particles of a different substance are involved. This process will involve the additional step of working out the number of moles of the substance we know the mass of using molar mass.
Reminder: Molar mass is the mass of one mole of a substance. It is calculated by finding the sum of the relative atomic masses of the elements present in the substance.
Question
A reaction between sodium oxide and carbon dioxide produces sodium carbonate.
How many formula units of sodium carbonate are produced from 62.0 grams of carbon dioxide?
Without conversion factors
Step 1: Write out and balance the equation that illustrates the chemical reaction.
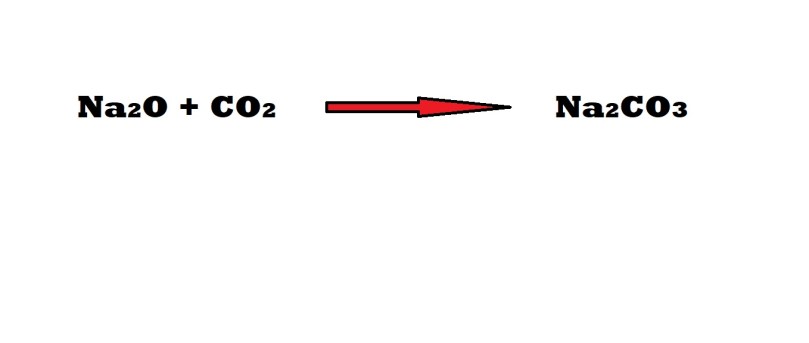
Conveniently, this equation is already balanced and therefore stoichiometric coefficients are not needed.
Step 2: Calculate the molar mass of carbon dioxide. We need to know this in order to work out how many moles of carbon dioxide are involved as a reactant using the known mass.
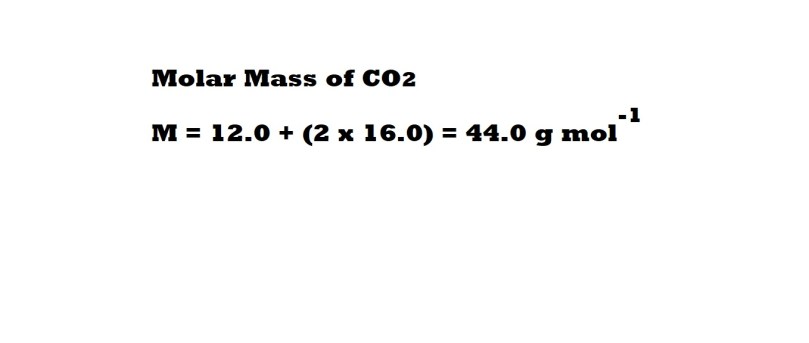
Step 3: Calculate the amount in moles of carbon dioxide involved as a reactant.
Reminder: Calculating the amount in moles involves dividing the mass in grams by the molar mass.
Step 4: Determine the mole ratio (stoichiometric ratio) between carbon dioxide and sodium carbonate.
The mole ratio of carbon dioxide to sodium carbonate is 1 mole to 1 mole.
Reminder: We don’t tend to write 1 as a stoichiometric coefficient in a chemical equation. The absence of a stoichiometric coefficient before a chemical formula means 1 mole of that substance is in the mole ratio.
Step 5: Due to the mole ratio between carbon dioxide and sodium carbonate being 1 mole to 1 mole, consider the amount in moles of sodium carbonate being produced to be equal to the number of moles of carbon dioxide acting as a reactant.
For illustrative purposes, we have multiplied the amount in moles of carbon dioxide by 1 to give the amount in moles of sodium carbonate. However, the values are equal because multiplying by 1 doesn’t change the value.
Step 6: Multiply the amount in moles of sodium carbonate produced by Avogadro’s number to determine the number of formula units produced.
Steps 3 – 6 are illustrated below.

With conversion factors
Steps 1 and 2 are the same as steps 1 and 2 in the method without conversion factors.
Step 3: Organise the calculation to include the given mass of carbon dioxide followed by three conversion factors: the first consisting of the molar mass of carbon dioxide and the equivalent value of 1 mole of carbon dioxide; the second illustrating the mole ratio between sodium carbonate and carbon dioxide and the third consisting of Avogadro’s number with the unit of formula units of sodium carbonate and the equivalent value of 1 mole of sodium carbonate.
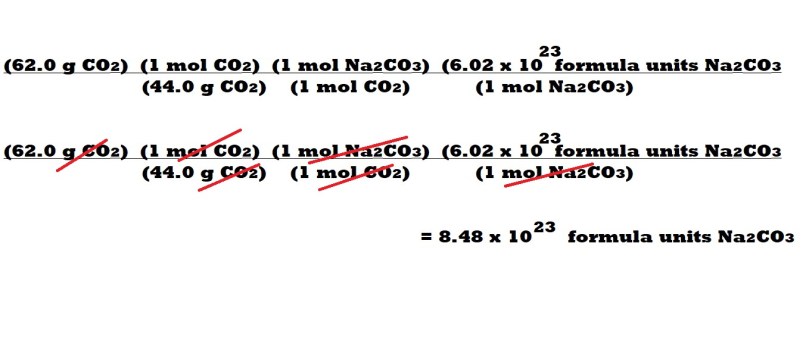
From left to right: The given mass of carbon dioxide; the molar mass of carbon dioxide as the denominator and the equivalent value of 1 mole of carbon dioxide as the numerator; the mole ratio between sodium carbonate and carbon dioxide of 1 mole to 1 mole; Avogadro’s number with the unit of formula units of sodium carbonate and the equivalent value of 1 mole of sodium carbonate.
The correct placement of the above variables allows us to cancel out the units we don’t want for our final answer and only leave the unit we want which is formula units of sodium carbonate.
We can then carry out the calculation and give our answer to three significant figures because the given value in the question (the mass of carbon dioxide) was given in three significant figures.
So there we go, we have worked out how to determine the number of particles of a substance that are involved either as a reactant or is a product of a chemical reaction.
In the next two tutorials we’ll be:
Identifying limiting and excess reactants.
Calculating the percentage yield and the atom economy of a chemical reaction.
Bye for now.
All images featured are my own.