In this first part of the third tutorial of the trio of tutorials on stoichiometry on this blog, we are going to be going through: what a limiting reactant is and how to identify one in a chemical reaction; how to calculate the amount of product produced using a certain amount of a limiting reactant and how to identify the excess reactant and work out how much excess is left over from a chemical reaction.
Note: Limiting and excess reactants can also be referred to as limiting and excess reagents.
What is a limiting reactant?
A limiting reactant is the substance that is completely used up when a reaction takes place. It is known as a ‘limiting’ reactant because it limits the reaction so that only a certain maximum amount of product can be produced.
Another way of thinking about a limiting reactant is that it’s usually the substance that you don’t have enough of, but that doesn’t necessarily mean it’s the substance that you have the least amount of. You can have reactions where the initial amount you have of the limiting reactant is more than the amount of other reactants – but nevertheless it’s the reactant that runs out first.
What is an excess reactant?
When the full amount of a limiting reactant is used up to produce the maximum amount of product possible, it is possible that you will have some amount of another reactant left over; this amount is known as excess and the substance is known as an excess reactant.
A typical question.
Let’s now look at a typical question that you could get that involves identifying the limiting and excess reactants in a chemical reaction and calculating the amount of product that can be produced using a certain amount of a limiting reactant.
Question
What is the maximum amount of calcium oxide that can be produced with 5.10 moles of calcium and 3.73 moles of oxygen? What is the limiting reactant? What is the excess reactant reactant and how much excess is left over?
You may not be given the balanced chemical equation in a test or exam and may instead have to write out and balance the equation.
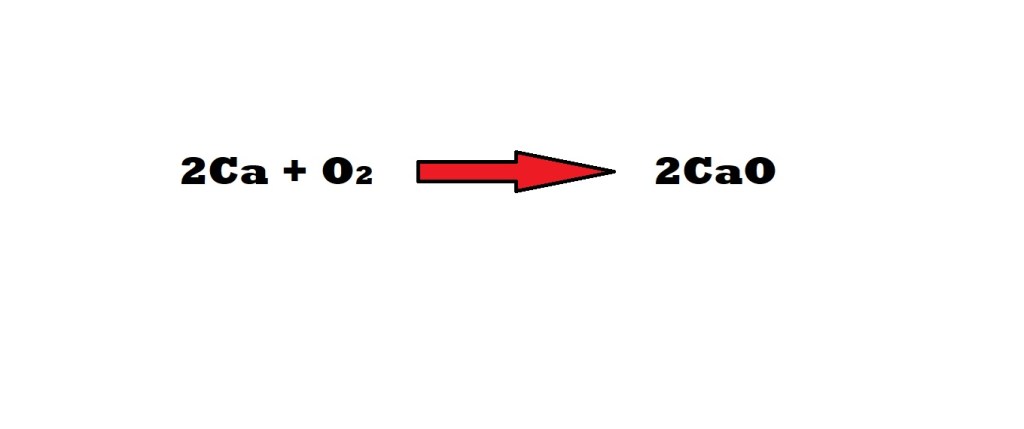
Step 1: Identify the limiting reactant.
To identify the limiting reactant, we need to work out how much of each reactant is needed to use up all of the other reactant in the reaction.
The amount of calcium needed to use up all of the oxygen:
To calculate the amount of calcium needed to use up all of the oxygen, we need to consider the mole ratio (also known as the stoichiometric ratio) between calcium and oxygen.
The mole ratio (stoichiometric ratio) in this part of the question refers to the number of moles of one reactant that reacts with a certain number of moles of another reactant. It is determined by stoichiometric coefficients – the numbers placed in front of chemical formulas to balance equations.
The stoichiometric ratio is within the conversion factor that the amount in moles of calcium is multiplied by in order to work out how much calcium is needed to use up all up the oxygen.
Reminder: The absence of a stoichiometric coefficient before a chemical formula in an equation means that there is 1 mole of that substance.
The mole ratio between calcium and oxygen according to the balanced chemical equation is 2 moles to 1 mole.

Amount of oxygen needed to use up all of the calcium:

Which is the limiting reactant?
Amount of calcium we have: 5.10 mol
Amount of calcium we need to use up all the oxygen: 7.46 mol
Amount of oxygen we have: 3.73 mol
Amount of oxygen we need to use up all the calcium: 2.55 mol
We have enough oxygen to use up all of the calcium, however we don’t have enough calcium to use up all of the oxygen.
Therefore, calcium is the limiting reactant.
Step 2: Calculate the maximum amount of product that can be produced using the limiting reactant.
Now that we have identified calcium as the limiting reactant, we now can work out the maximum amount of calcium oxide that can be produced with the amount of calcium we have and the amount of oxygen we have.
To do this, we must multiply the amount of calcium that we have by a conversion factor that consists of the mole ratio (stoichiometric ratio) between the limiting reactant – calcium – and the product of the reaction – calcium oxide.
According to the balanced chemical equation, the mole ratio between calcium and calcium oxide is 2 moles to 2 moles.
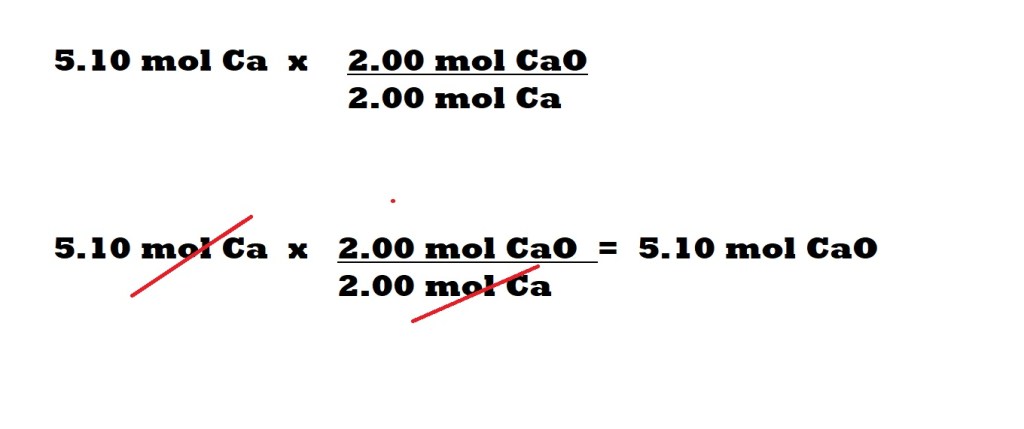
The maximum amount of calcium oxide that can be produced with the given amounts of calcium and oxygen is 5.10 moles.
Step 3: Identify the excess reactant and calculate the excess.
The final step to answer this question is to identify the excess reactant and calculate the amount of excess that’s left over after the reaction stops when all of the limiting reactant is used up.
We know that calcium is the limiting reactant because we don’t have enough of it to use up all of the oxygen, but we also know that we have enough oxygen to use up all of the calcium and that we have oxygen left over.
Amount of oxygen we need to use up all of the calcium : 2.55 moles
Amount of oxygen we have: 3.73 moles
Therefore, oxygen is the excess reactant.
So now we can work out the amount of excess of oxygen that’s left over; this involves using the following equation:

So using the equation above we can calculate the amount of excess oxygen left over as follows:

So we have worked out that:
Calcium is the limiting reactant.
The maximum amount of calcium oxide that can be produced is 5.18 moles.
Oxygen is the excess reactant and 1.18 moles are left over.
A more advanced question.
Okay let’s have a look at a question that’s a little trickier than the previous one.
What is the maximum mass of hydrochloric acid (HCl) that can be produced with 43.0 grams of hydrogen and 98.0 grams of oxygen? Which reactant is the limiting reactant? Which reactant is in excess and what is the mass of the excess?
The difference between this question and the previous one is that we are given the masses (in grams) of the reactants rather than the amounts in moles and we have to work out the maximum mass of product that can be produced rather than the amount in moles. This will involve some additional steps that weren’t required for the previous question.
Let’s go through this step-by-step.
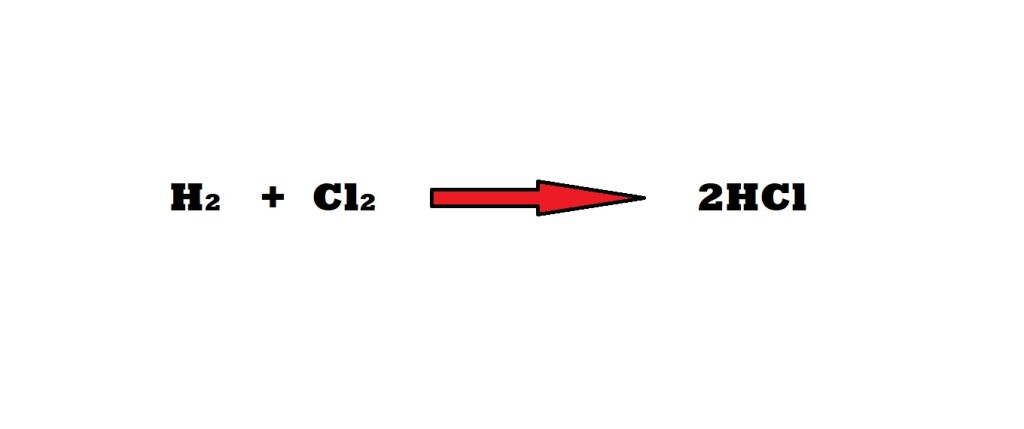
Step 1: Convert the masses in grams of the reactants involved into amounts in moles.
The first step involves converting both reactants from mass in grams to amount in moles. This involves using the following equation:

Remember: Molar mass is the mass of one mole of a chemical substance. Molar mass is represented by the symbol M.
Conversion Factor Method
Remember: When using the conversion factor method for converting between grams and moles we replace mol-1 in the unit of molar mass with the chemical formula of the substance involved.


Without Conversion Factors


The amounts (in moles) of hydrogen and chlorine have been rounded to three significant figures because this is the number of significant figures present in the given value with the fewest significant figures.
Step 2: Identify the limiting reactant.
Now that we know the amounts (in moles) of hydrogen and chlorine involved, we can now work out which reactant is the limiting reactant. Once again this will involve calculating the amount of each reactant needed to use up all of the other reactant in the reaction.
Remember: The conversion factor for this part of the question consists of the mole ratio (stoichiometric ratio) between the two reactants according to the balanced chemical equation.
The mole ratio between the reactants of hydrogen and chlorine is 1 mole to 1 mole.
Amount of chlorine needed to use up all of the hydrogen:

Amount of hydrogen needed to use up all of the chlorine:
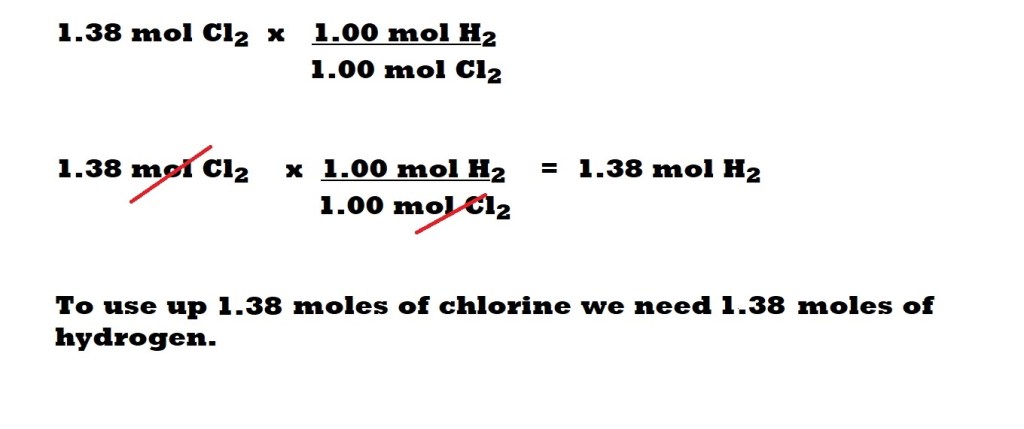
Which is the limiting reactant?
Amount of hydrogen we have: 21.3 mol
Amount of hydrogen we need to use up all of the chlorine: 1.38 mol
Amount of chlorine we have: 1.38 mol
Amount of chlorine we need to use up all of the hydrogen: 21.3 mol
We have enough hydrogen to use up all of the chlorine, but we don’t have enough chlorine to use up all of the hydrogen.
Therefore, chlorine is the limiting reactant.
Step 3: Work out the maximum amount of product produced using the given amount of limiting reactant.
Now that we have identified chlorine as the limiting reactant, we can work out the maximum amount of hydrochloric acid that can be produced with the given amount of chlorine.
Remember: The conversion factor for this part of the question consists of the mole ratio between the limiting reactant and the product of the reaction according to the balanced chemical equation.
The mole ratio between chlorine – the limiting reactant – and hydrochloric acid – the product – is 1 mole to 2 moles.

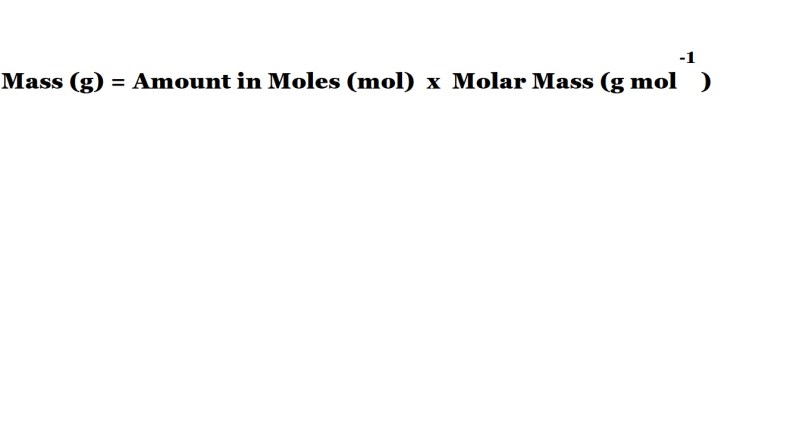
Step 4: Convert the maximum amount (in moles) of the product to the maximum mass (in grams).
Now that we know the maximum amount in moles of hydrochloric acid that can be produced, we can now convert to the maximum mass in grams of hydrochloric acid that can be produced via the reaction.
This can be done via the use of the following equation:
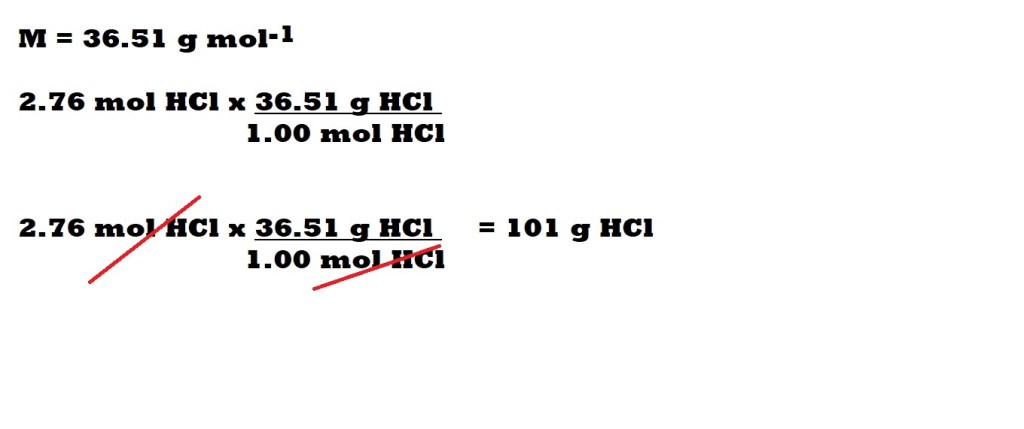
Conversion Factor Method
Without Conversion Factors
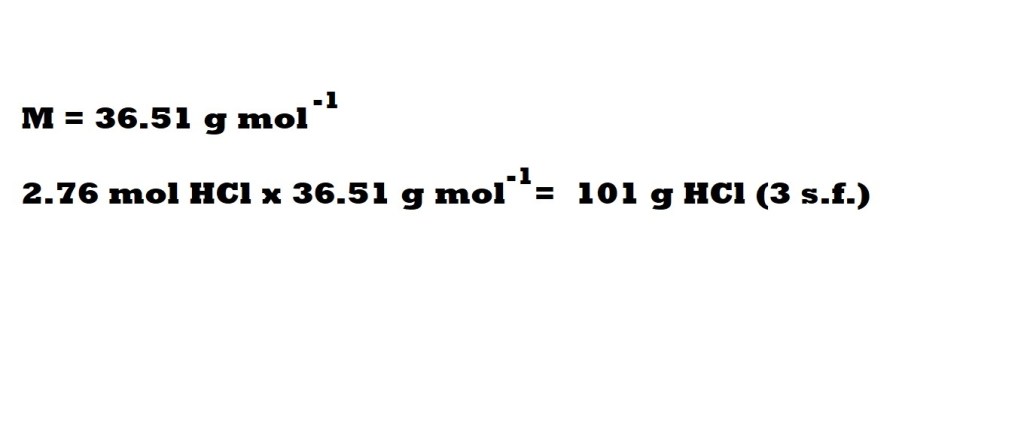
We have rounded the maximum mass of hydrochloric acid produced to three significant figures because the masses of hydrogen and chlorine are given in three significant figures in the question.
So the maximum mass of hydrochloric acid that can be produced is 101 g.
Step 5: Identify the excess reactant and calculate the amount of excess in moles.
So now we know that chlorine is the limiting reactant, we can now identify hydrogen as the excess reactant because we have enough hydrogen to use up all the chlorine and there is some left over.
Amount of hydrogen we have: 21.3 mol
Amount of hydrogen we need to use up all the chlorine: 1.38 mol
Hydrogen is the excess reactant.

So using the above equation, we can work out the amount in moles of hydrogen left over.

Step 6: Convert the amount of excess in moles to mass in grams.
So now we know the amount in moles of the excess hydrogen left over, we can now convert to the mass of hydrogen left over.
This is done with the following equation:

Conversion Factor Method
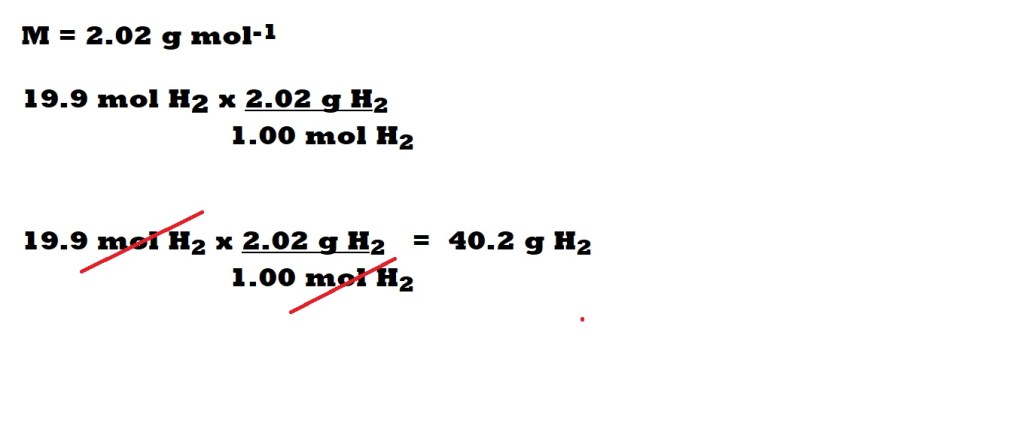
Without Conversion Factors
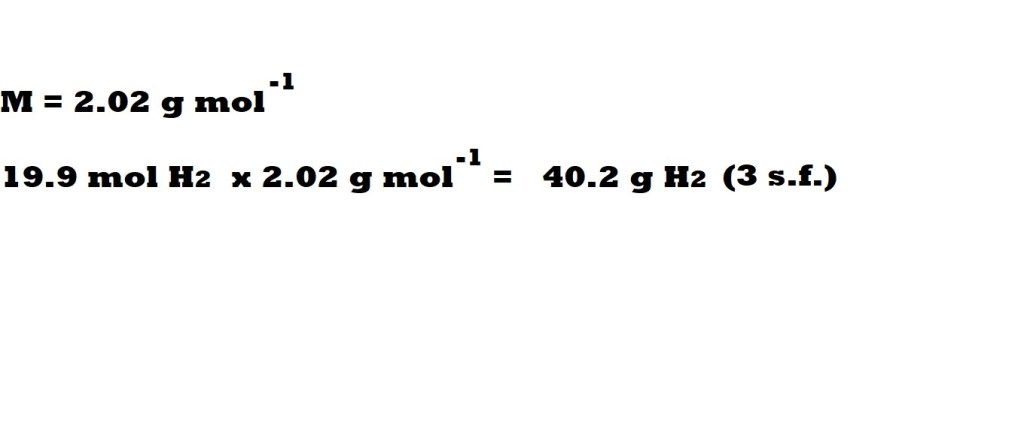
We have rounded the mass of the excess of hydrogen to three significant figures because the masses of the reactants are given in three significant figures in the question.
So we have worked out that:
Chlorine is the limiting reactant.
The maximum mass of hydrochloric acid that can be produced is 101 grams.
Hydrogen is the excess reactant and the mass of the excess is 40.2 grams.
So there we go, we have worked out how to identify limiting and excess reactants and how to calculate the maximum amount or mass of a product that can be produced.
In the next tutorial, we will be going over how to calculate the percentage yield and atom economy of a chemical reaction.
All images featured are my own.