In this tutorial, we are going to go through how to calculate the percentage yield and the atom economy of a reaction.
What do we mean by ‘yield’?
The term ‘yield’ refers to the production of some form of matter and when we’re figuring out the yield of a reaction we are usually referring to how much of a product is produced following a forward reaction.
Theoretical Yield and Actual Yield
The calculation of the percentage yield involves two values: the theoretical yield and the actual yield (also known as the experimental yield).
The theoretical yield is the maximum mass of a specific product that can be produced if all reactants are converted into products.
However, it’s often the case that the maximum possible yield of a reaction is not produced and the mass of a product that is actually produced following a reaction is referred to as the actual yield or the experimental yield.
The percentage yield indicates – via a percentage value – the proportion of the theoretical yield that is produced in the form of the experimental yield. For instance, if the theoretical yield is 60g and the experimental yield is 30g then the percentage yield will be 50% because half of the maximum possible mass (the theoretical yield) of a product is actually produced.
A high percentage yield indicates that a high proportion of the theoretical yield has been produced and therefore a reaction can be considered efficient.
Ideally – especially in industry – every reaction would have a 100% percentage yield because all reactants and resources would be efficiently used. But this is rare.
Calculating the Percentage Yield
To calculate the percentage yield of a reaction, we need the following equation:

Question:
A combustion reaction between methane gas and oxygen gas leads to the production of carbon dioxide gas and water.
If 19.0g of methane (CH4) combusts following a reaction with oxygen (O2) and 12.0g of carbon dioxide (CO2) is produced, what would be the percentage yield of carbon dioxide?
The first thing we have to consider is the balanced chemical equation that represents the reaction.
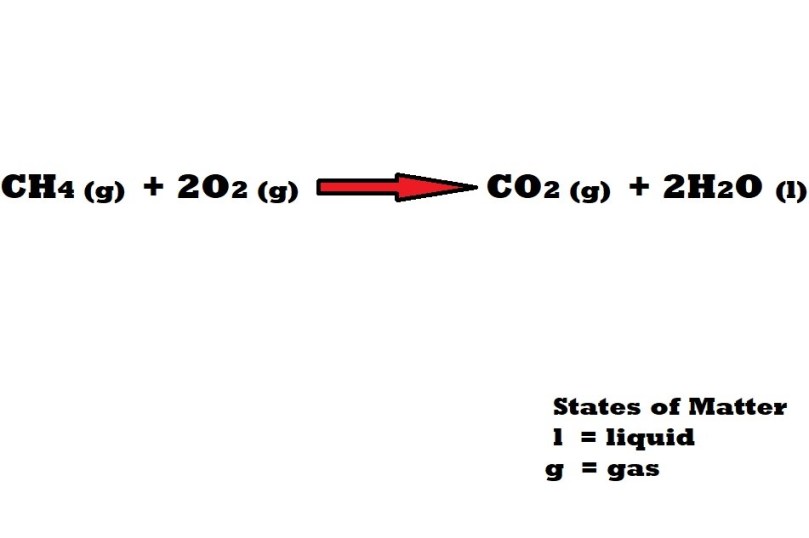
Note: You may be provided with a balanced chemical equation for the reaction in a question or you may have to balance out an equation by including stoichiometric coefficients yourself.
We need to use the balanced chemical equation to work out the mole ratio (stoichiometric ratio) between the substance we are given the mass of in the question (methane) and the substance that we are being asked to work out the percentage yield of (carbon dioxide).
We then have to work out the molar masses of both methane and carbon dioxide. We then use the given mass of methane; the molar mass of methane; the mole ratio between methane and carbon dioxide and the molar mass of carbon dioxide to calculate the maximum mass of carbon dioxide that can theoretically be produced – thereby calculating the theoretical yield.
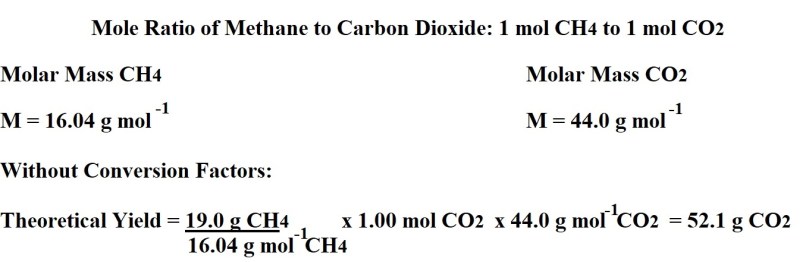
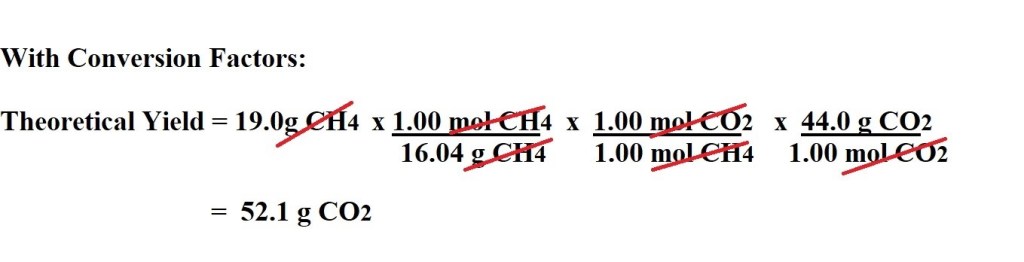
If all reactants are converted into products, 19.0g of methane produces 52.1g of carbon dioxide therefore the theoretical yield of carbon dioxide is 52.1g.
Now we know that the theoretical yield of carbon dioxide is 52.1 g, we can now take that value and the given value representing the actual yield (experimental yield) of carbon dioxide – which is 12.0 g – and input them into the equation to calculate the percentage yield.
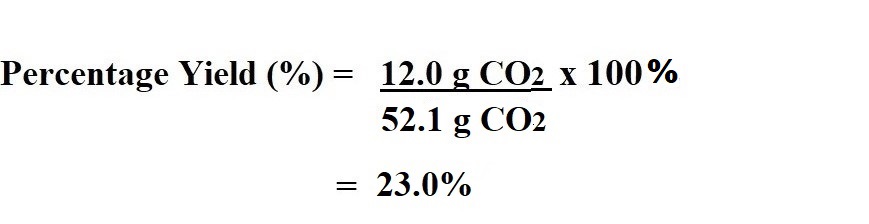
So we have worked out that the percentage yield for this reaction is 23%, which is a quite low and therefore the reaction could be considered inefficient.
Atom Economy: What is the Atom Economy of a Chemical Reaction?
When examining the efficiency of a chemical reaction it’s also important to measure how much waste is created in the form of waste products (also known as by-products) when trying to create a desired product. This will allow us to ascertain how sustainable a reaction is environmentally and economically (especially if it’s part of an industrial process).
The consideration of how much waste results from a chemical reaction and the minimisation of waste is fundamental in an area of chemistry known as Green Chemistry and is measured via a value known as the reaction’s atom economy.
Calculating the Atom Economy of a Reaction
To calculate the atom economy of a reaction we can use the following equation:
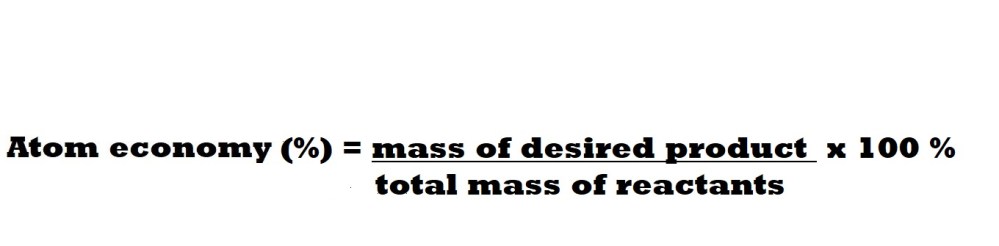
Note: In some textbooks or other resources you may find an equation in which atom economy is calculated by dividing the mass of a desired product by the total mass of products rather than the total mass of reactants. This will lead to the same answer that you would get if you used the equation above because the total mass of reactants and total mass of products are equal in value because of the law of conservation of mass.
However, the most common method used in an atom economy calculation is illustrated in
the equation above.
The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.
For example, if the mass of the desired product was only a quarter of the total mass of the reactants, such as would be the case if 10g of the desired product was produced and the total mass of the reactants was 40g, the atom economy of the reaction would be 25%.
A higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Therefore, a higher atom economy indicates a more efficient reaction.
Question:
A reaction between fluorine (F2) and potassium bromide (KBr) leads to the production of potassium fluoride (KF) and bromine (Br2). What would be the atom economy of this reaction if potassium fluoride is the desired product?
In this question there are no given masses (in grams) or amounts (in moles), so how can we answer this question? Well, in this situation, the stoichiometric coefficients in the balanced chemical equation for the reaction are really handy.
We have to use the stoichiometric coefficients in the equation to work out how many moles of the reactants and the desired product are involved in the reaction. We then calculate the masses of the reactants and the desired product by multiplying the number of moles of each substance by each one’s corresponding molar mass.
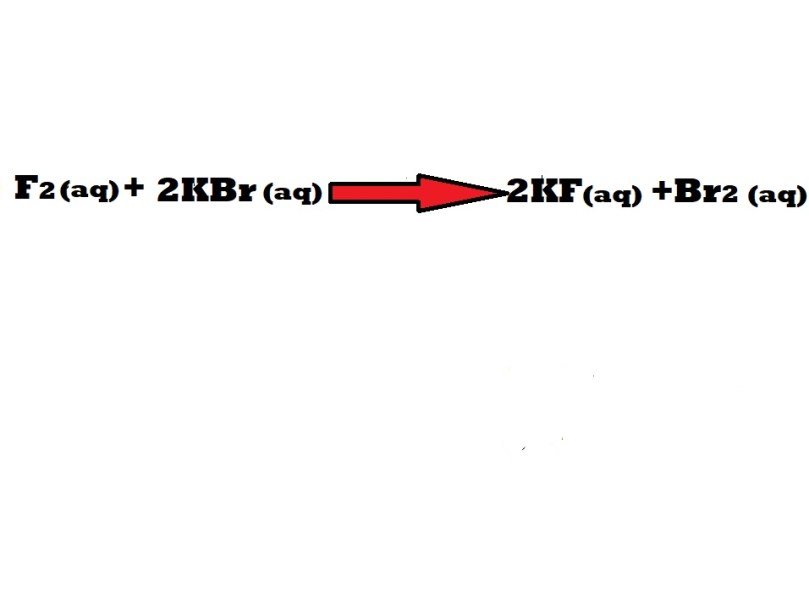
Note: You may be provided with a balanced chemical equation for the reaction involved in a question or you may have to balance an equation by including stoichiometric coefficients yourself.
According to the stoichiometric coefficients in the balanced chemical equation, when 1 mole of fluorine reacts with 2 moles of potassium bromide 2 moles of potassium fluoride are produced.

The mass of the desired product – which is potassium fluoride – produced following the reaction is 116.2g.
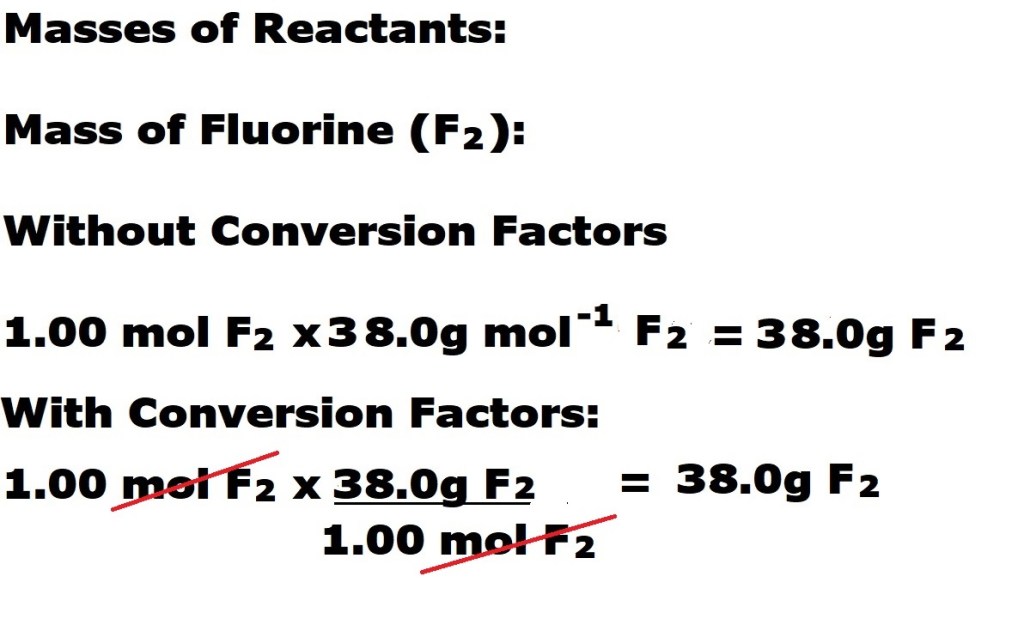

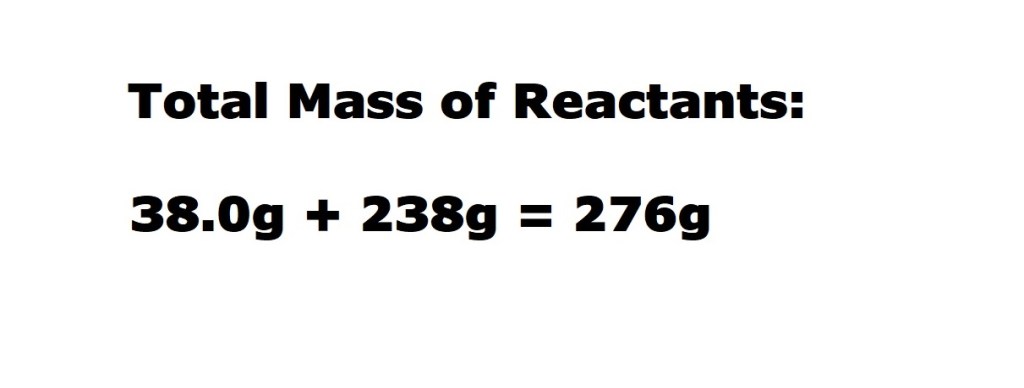
Now we know both the total mass of the reactants (fluorine and potassium bromide) and the mass of the desired product (potassium fluoride), we can now input those values into the equation to calculate the reaction’s atom economy.
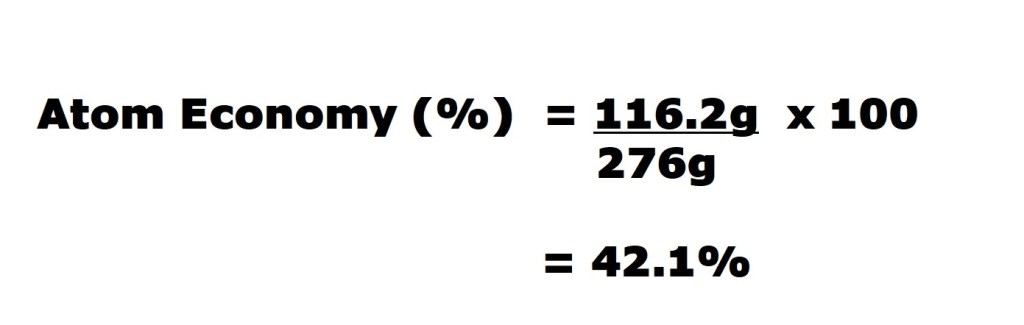
So we have worked out that the atom economy of the reaction is 42.1%, which isn’t great with regards to efficiency.
So there we go, we now know how to define and calculate the percentage yield and atom economy of a chemical reaction.
In the next few tutorials, we are going to tackle gas stoichiometry.
Bye for now.