
When it comes to working out the mass, amount or volume of a gaseous substance in isolation or in a reaction, you have to consider the properties of temperature and pressure.
The first part of this tutorial on gas stoichiometry will focus on calculating the volume (in dm3 or litres), amount in moles and mass in grams of gaseous substances in isolation or within a reaction at standard temperature and pressure (STP).
We will look at some examples of questions and we will breakdown how to work out and answer each question step-by-step.
What is STP?
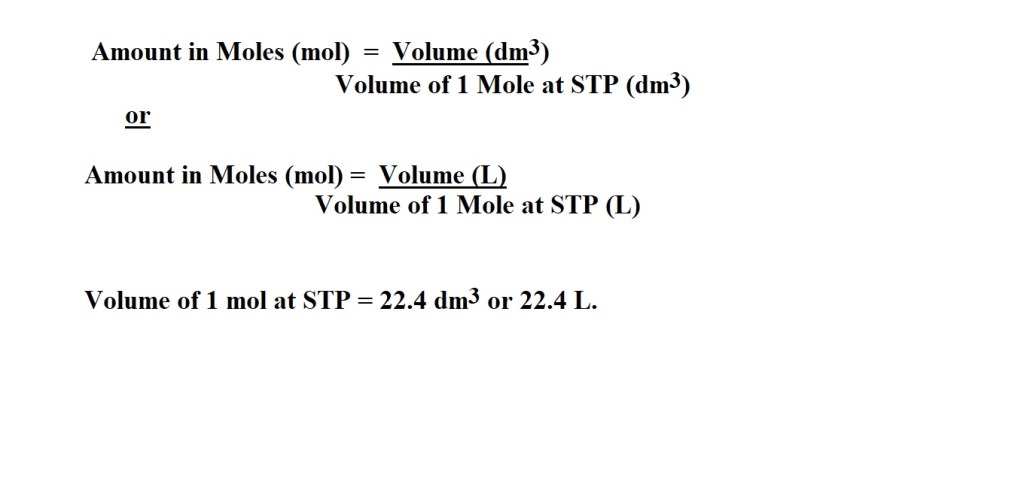
STP describes the conditions of an environment in which 1 mole of a gas occupies a volume of 22.4 dm3. or 22.4 L.
However, the temperature of the environment must be 0oC (273.15 K) and it must have a pressure of 1 atmosphere (101.325 kPa).
Note:. Please check with your teacher, lecturer or tutor which unit you need to use for your course to express volume (dm3 or litres).
Let’s look at some questions.
Calculating the Volume of Moles of a Gas using the STP Method
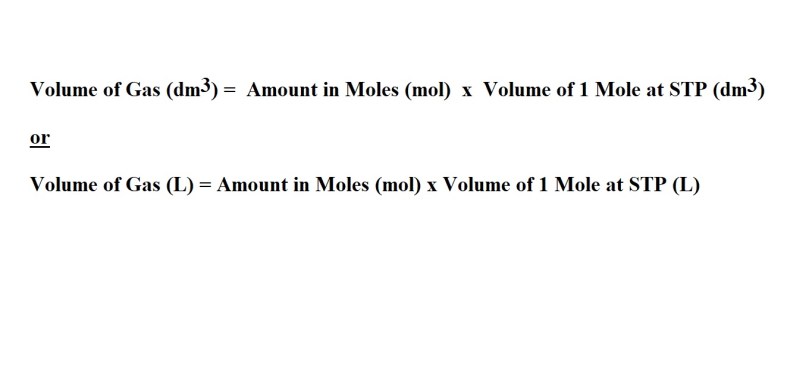
These are the equations you will need – depending on which unit you are going to use to express the volume of the gas.
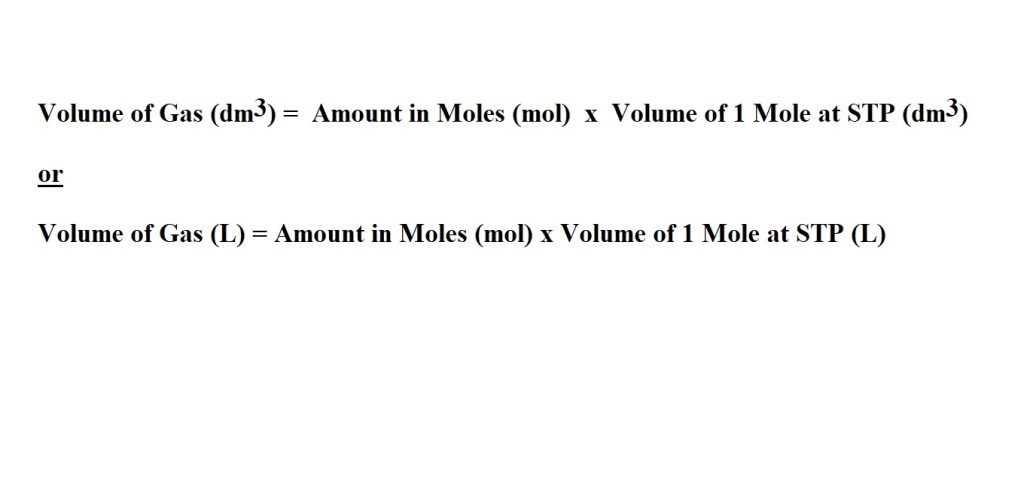
Question: What is the volume in dm3 of 4.10 moles of NO2 at STP?
Working:
We can do this in two ways – with a conversion factor to cancel out units we don’t want and without a conversion factor.

Note: Include the same number of significant figures in your final answer as there are in the value in the question with the fewest significant figures.
We take the value in moles and multiply it by the known volume of 1 mole of a gas at STP which is 22.4 dm3.
We can then divide by 1 mole of nitrogen dioxide (the equivalent value as it’s based on the volume of 1 mole of a gas at STP) to allow us to cancel out the unit of mol NO2 and leave us with the unit we want for our answer which is dm3 NO2.
This conversion factor method is required for some specifications but not for others. Please check with your teacher, tutor or lecturer if this method is required for your course.

Answer: 91.8 dm3 NO2 (to 3 significant figures)
Let’s look at a question involving a reaction:
Question: What volume of ammonia is produced if 7.80 dm3 of hydrogen gas reacts with excess nitrogen at STP?
This kind of question requires a few steps.
Workings:
1) Write a balanced chemical equation that represents the reaction.
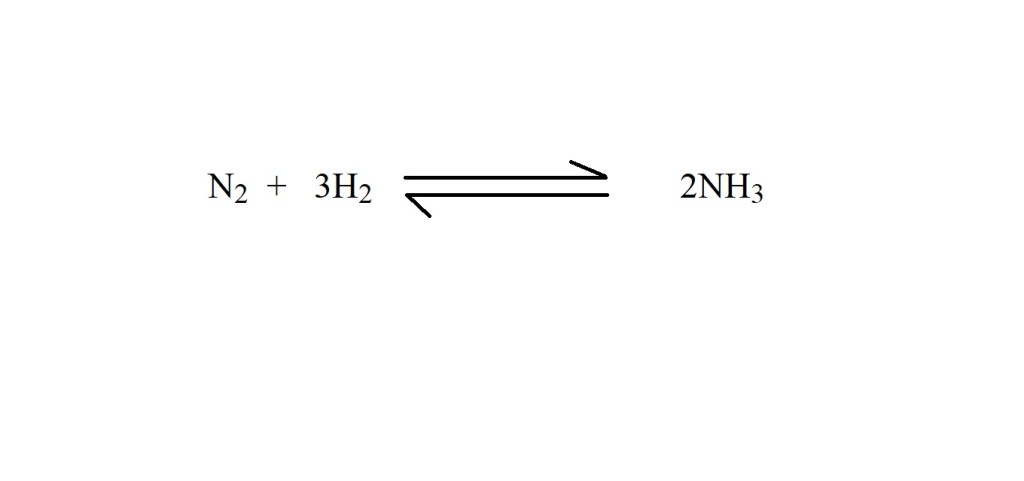
2) Convert the volume of hydrogen into amount in moles at STP. This can be done with the following equation:
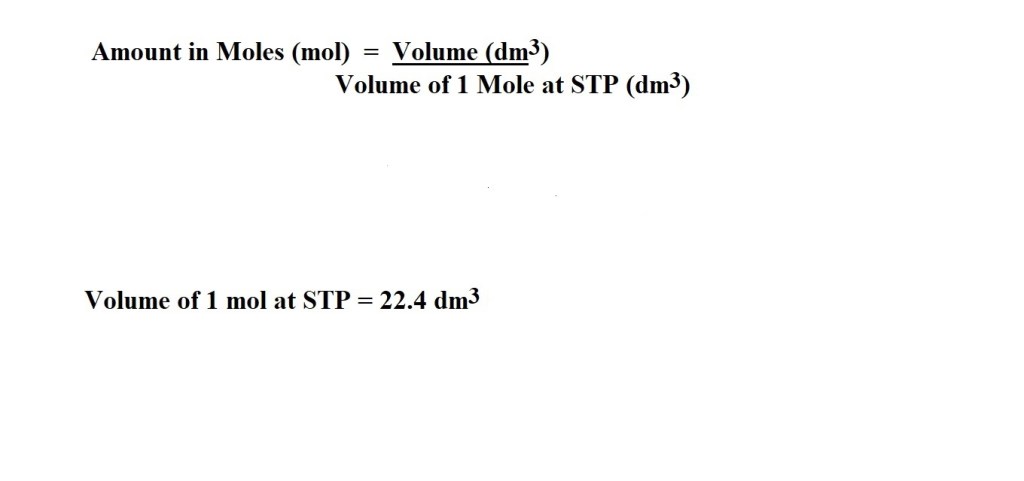
This can be done with the use of a conversion factor and without a conversion factor.
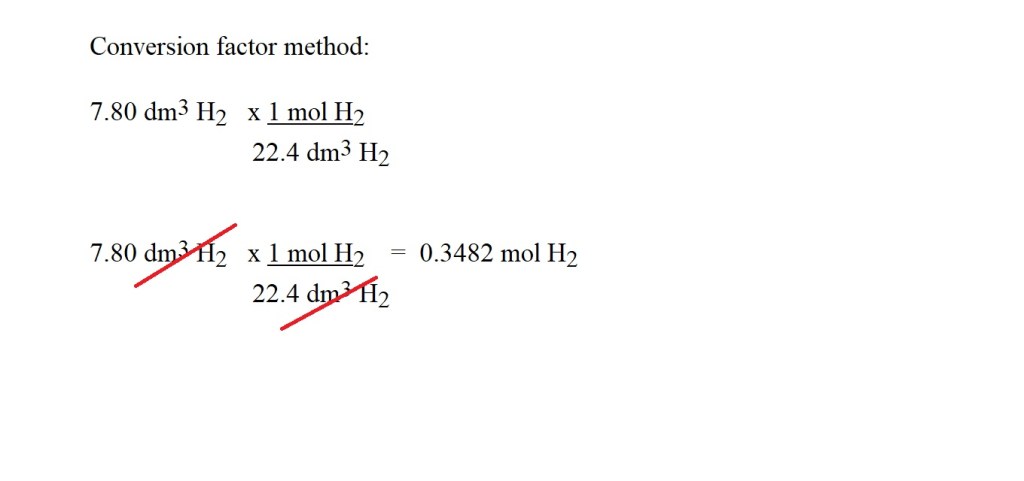

Note: The amount in moles has been given to four significant figures because this is not the final answer and we want to avoid rounding to too few significant figures in the middle of the calculation as this may lead to inaccuracy in your final answer.
I would advise going for four significant figures for amount in moles if it’s not the final answer to the question.
3) Work out the stoichiometric ratio (mole ratio) for the two substances in the question – which is hydrogen and ammonia.
Reminder: The stoichiometric ratio – also known as the mole ratio – is a ratio that represents the number of moles of one substance to the number of moles of another substance. This could show how many moles of one particular substance is produced with a certain number of moles of another substance as a reactant or the number of moles of one reactant that reacts with a certain number of moles of another reactant.
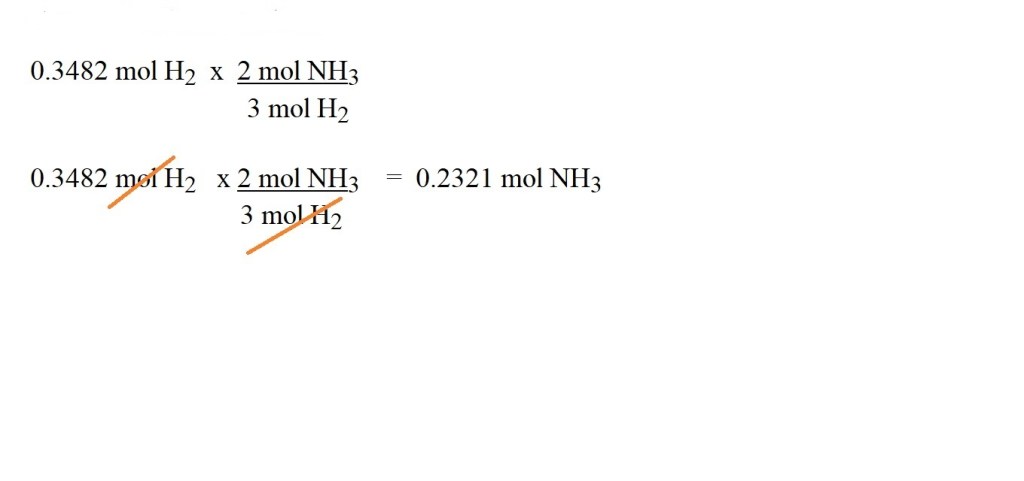
According to the balanced chemical equation, 3 moles of hydrogen reacts to produce 2 moles of ammonia – therefore the mole ratio is 3 moles of hydrogen to 2 moles of ammonia.
4) Use the mole ratio (stoichiometric ratio) to convert between the amount in moles of hydrogen gas to the amount in moles of ammonia gas.
We should cancel out the units we don’t want for our answer. We can do this by multiplying by a conversion factor (a fraction) that includes the number of moles of the gas in the mole ratio we want to convert from as the denominator and the number of moles of the gas in the mole ratio we want to convert to as the numerator.
This then leads to our answer which we must give in the correct units of mol NH3.
5) Convert from moles of ammonia to the volume of ammonia produced.
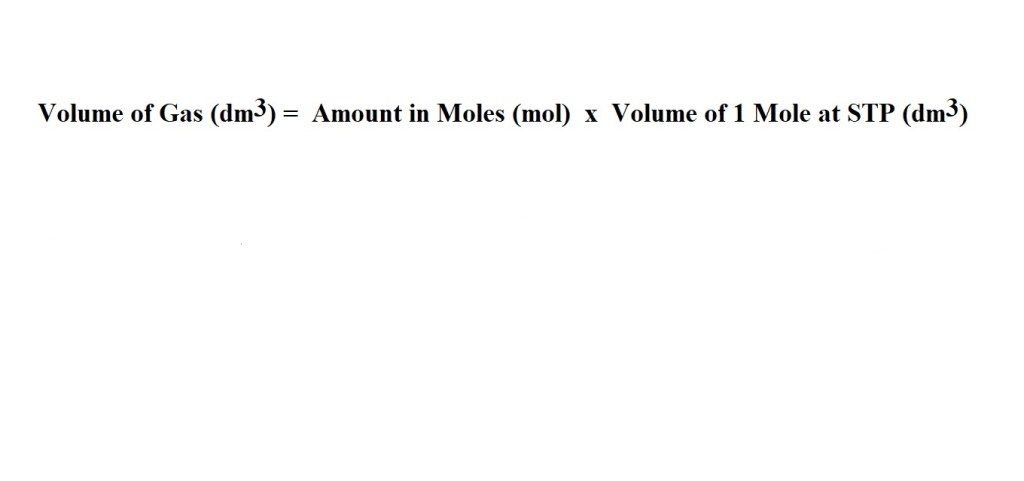
This can be done with a conversion factor to cancel out the units we don’t want for our answer:

Alternatively, it can be done without a conversion factor:

Answer: 5.20 dm3 NH3 (to 3 significant figures).
Calculating the Volume of a Mass of a Gas using the STP Method
You’ll need two equations for two separate steps.
The first step involves converting from mass in grams to amount in moles.
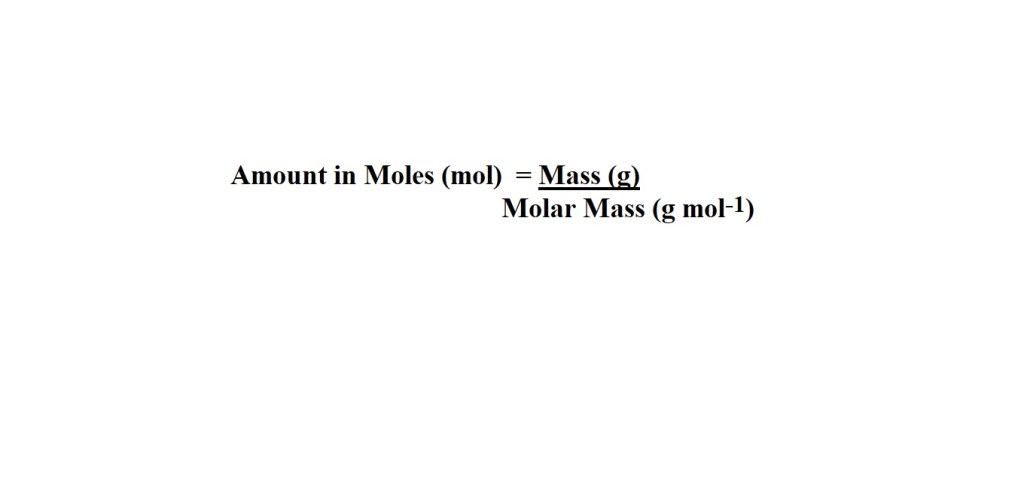
Reminder: Molar mass is the mass of one mole of a substance and is the sum of the relative atomic masses of the present atoms.
The second step involves converting from amount in moles to volume.
Question: What is the volume in litres (L) of 34.0g of CH4 at STP?
Working:
1) Firstly, convert the mass of methane into amount in moles.

This can be done in two ways – one involving a conversion factor to cancel out the unit of g CH4 :
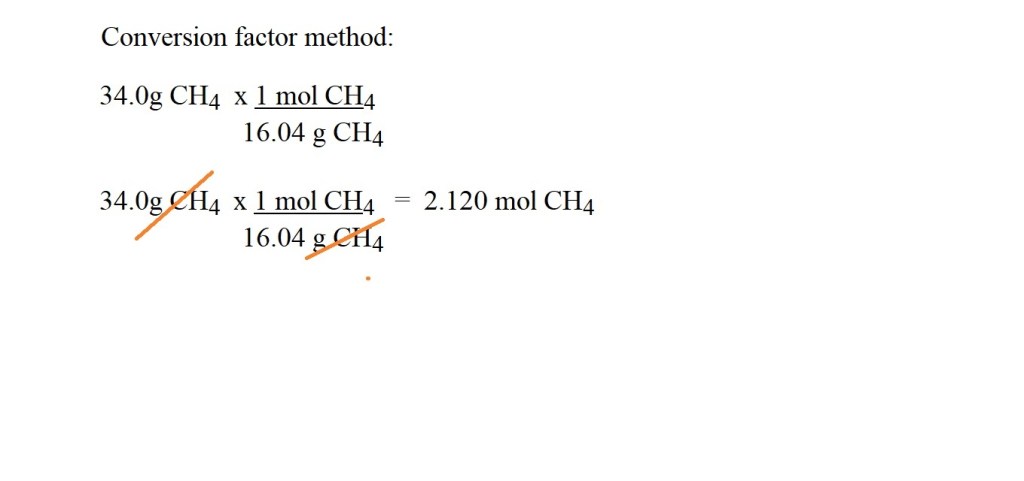
Reminder: 1 mol CH4 is an equivalent value for 16.04g CH4 in the conversion factor because the molar mass is the mass of one mole of the gas.
Alternatively, a non-conversion factor method can be used:

I would advise going for four significant figures for amount in moles if it’s not the final answer to the question.
2) Next we find the volume of methane according to the number of moles we have by multiplying by 22.4 L.
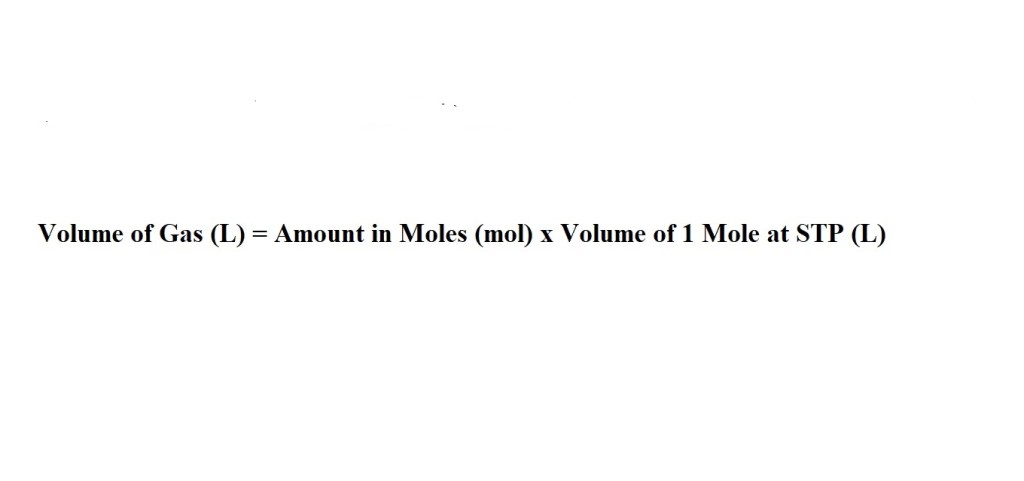
This can also be done with a conversion factor:
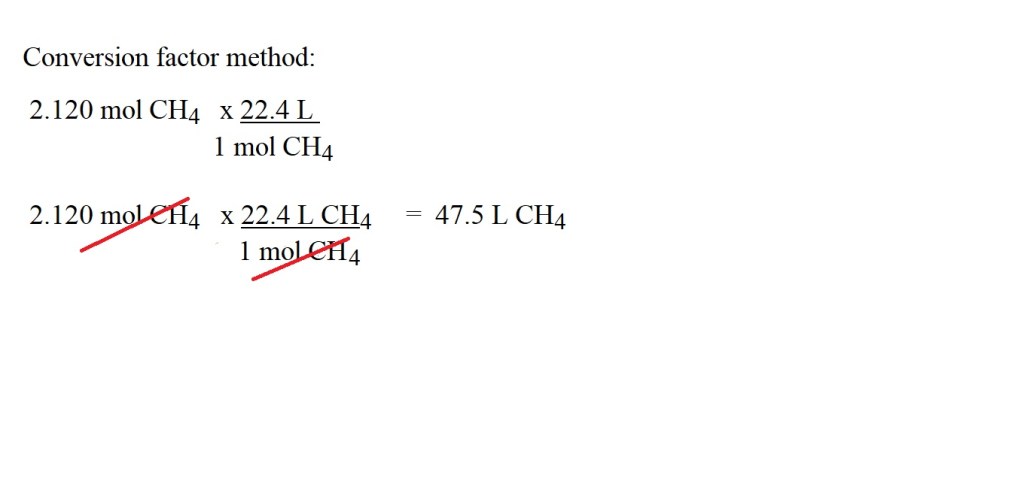
Alternatively, a non-conversion factor can be used:

Answer: 47.5 L CH4 (3 significant figures)
Let’s look at a question involving a reaction:
Question: What is the volume in dm3 of hydrogen fluoride gas (HF) produced from 98.0g of fluorine gas reacting with excess hydrogen gas?
Working:
Let’s look at this step-by-step once again.
1) Write out a balanced chemical equation that represents the reaction. (You may have to write it yourself in a test or exam.)
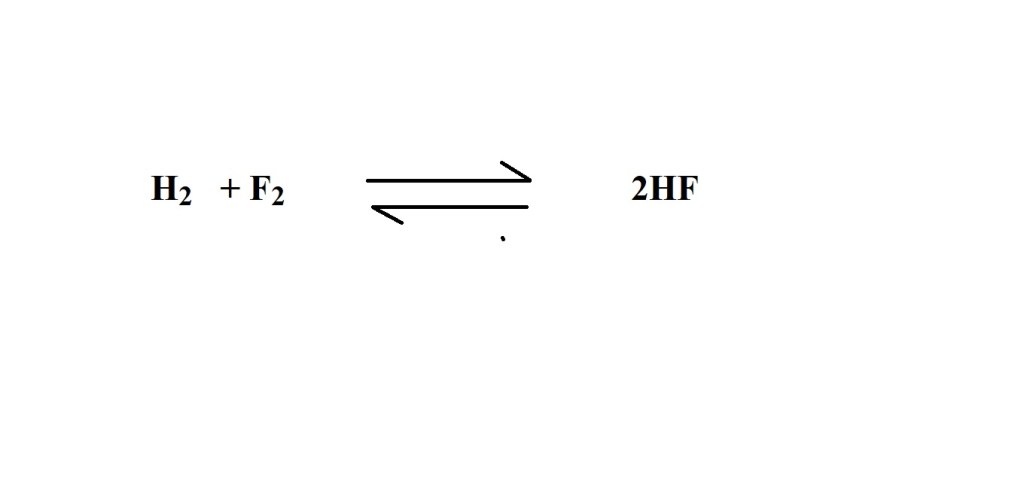
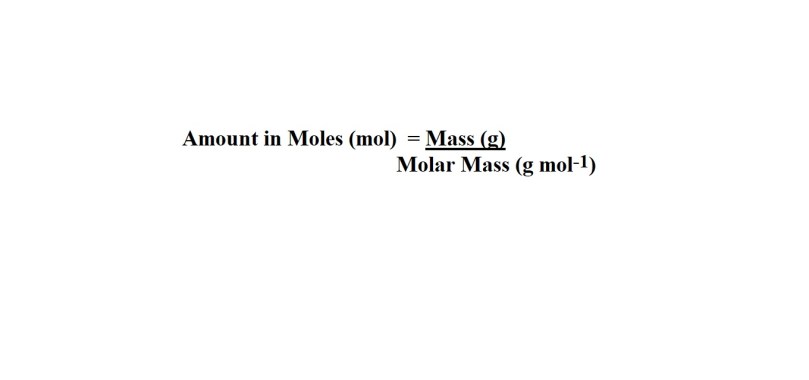
2) Convert the given mass of hydrogen fluoride (HF) into amount in moles.


3) Work out from the balanced equation what the stoichiometric ratio (mole ratio) is for fluorine to hydrogen fluoride.

According to the balanced equation 1 mole of fluorine gas reacts during the production of 2 moles of hydrogen fluoride so the mole ratio is 1 mole of fluorine to 2 moles of hydrogen fluoride.
4) Use the stoichiometric ratio (mole ratio) to convert the amount in moles of hydrogen fluoride into amount in moles of fluorine gas.
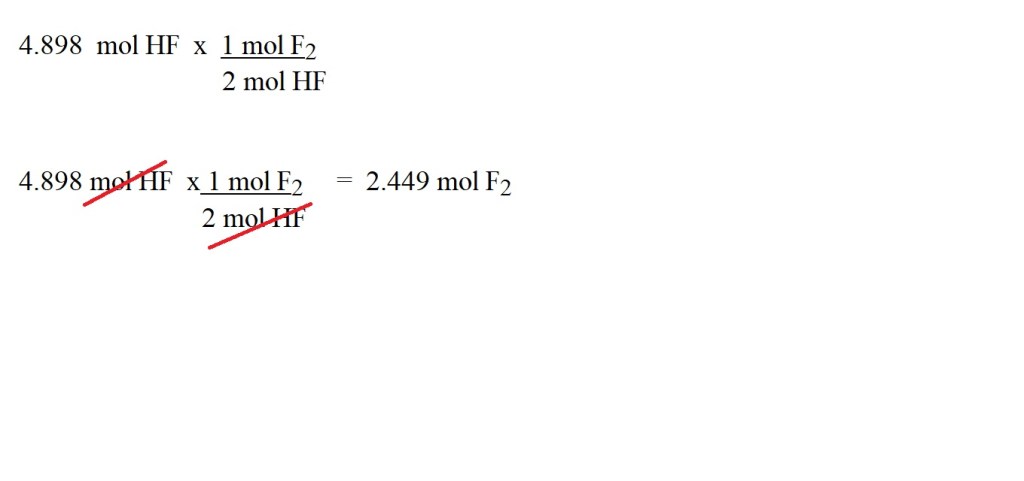
I would advise going for four significant figures for amount in moles if it’s not the final answer to the question.
5) Convert from the amount in moles of fluorine gas to volume in dm3.
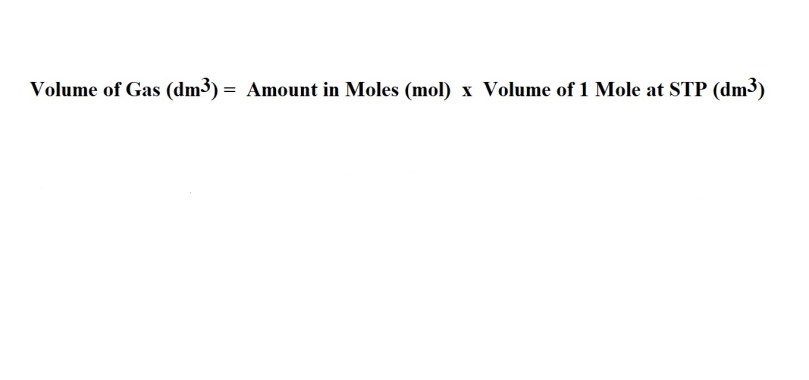


Answer: 54.9 dm3 (to 3 significant figures).
Calculating the Amount in Moles of a Gas using Volume and the STP Method
Some equations you’ll need:
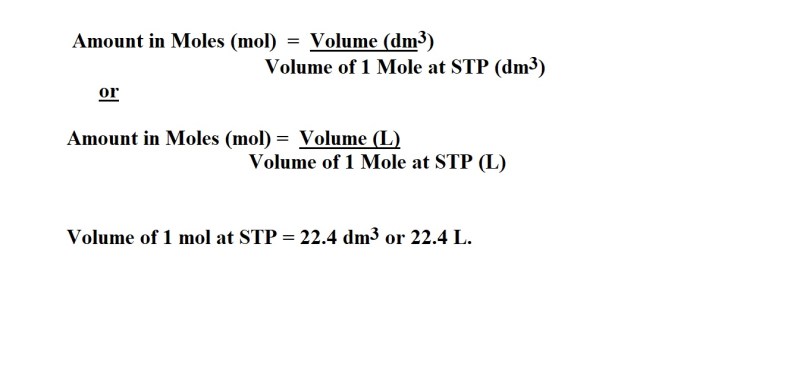
We’ve carried out the necessary steps for questions already, however it’s important to know when to use certain steps and when not to use certain steps in particular questions.
Question: How many moles are present in 6.80 dm3 of SO3 at STP?
Working:
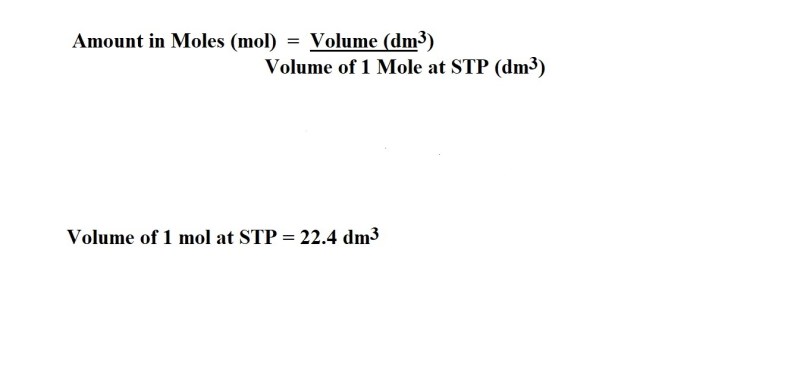
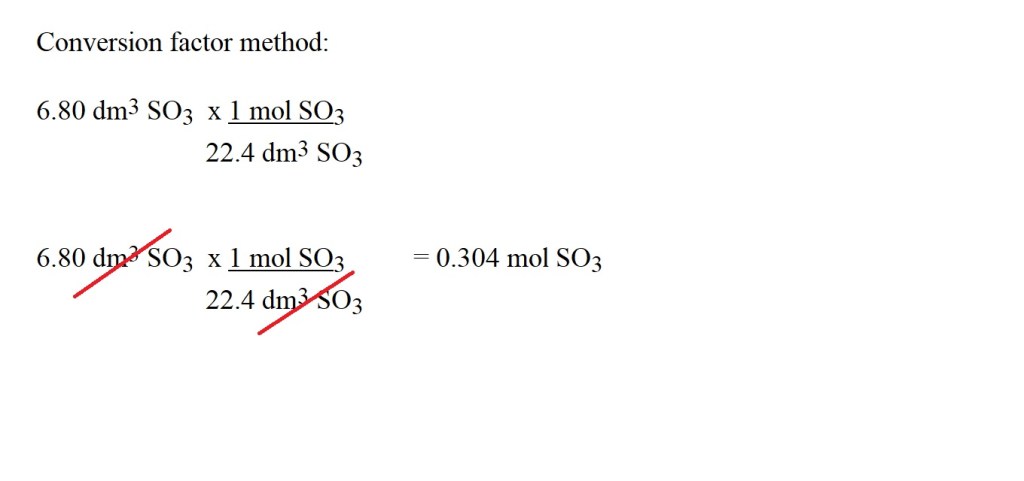

Answer: 0.304 mol SO3 (3 significant figures)
Remember: The final answer to this question is an amount in moles and therefore it should have the same number of significant figures as there are in the value in the question with the fewest significant figures.
Let’s look at a reaction:
Question: How many moles of iodine react with excess hydrogen to produce 9.85 L of hydrogen iodide gas?
This is another multiple step process but let’s break it down:
1) Write out a balanced chemical equation that represents the reaction (if it’s not already provided).
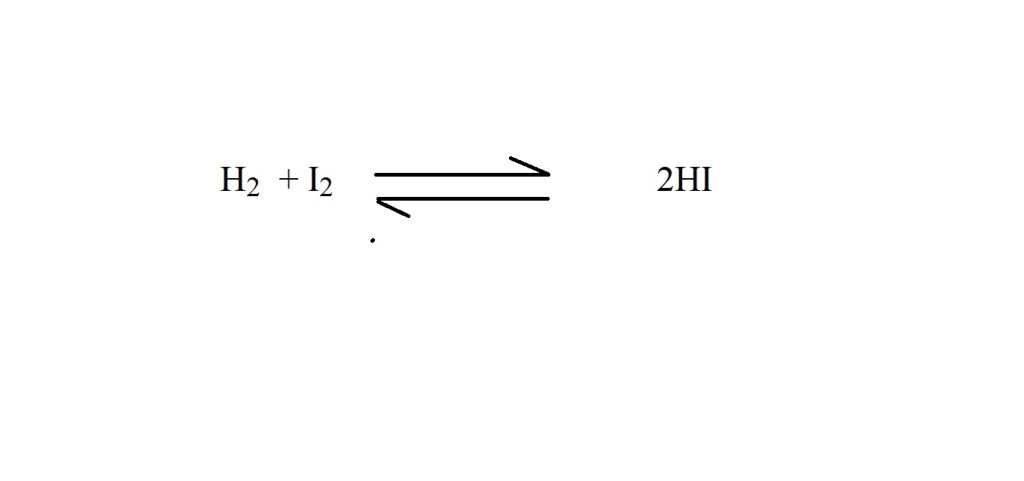
2) Convert the volume of hydrogen iodide gas into amount in moles.
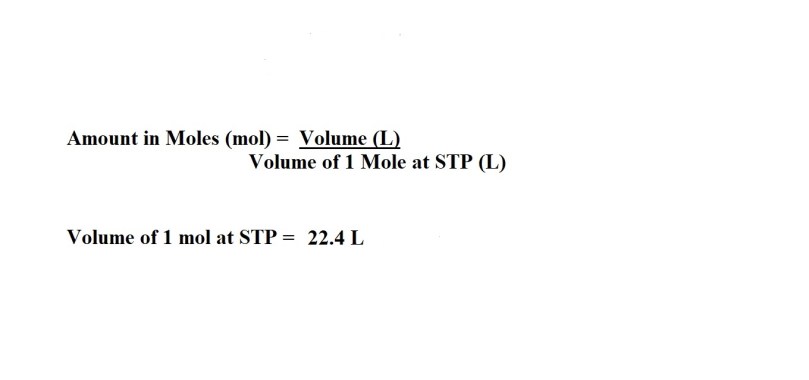
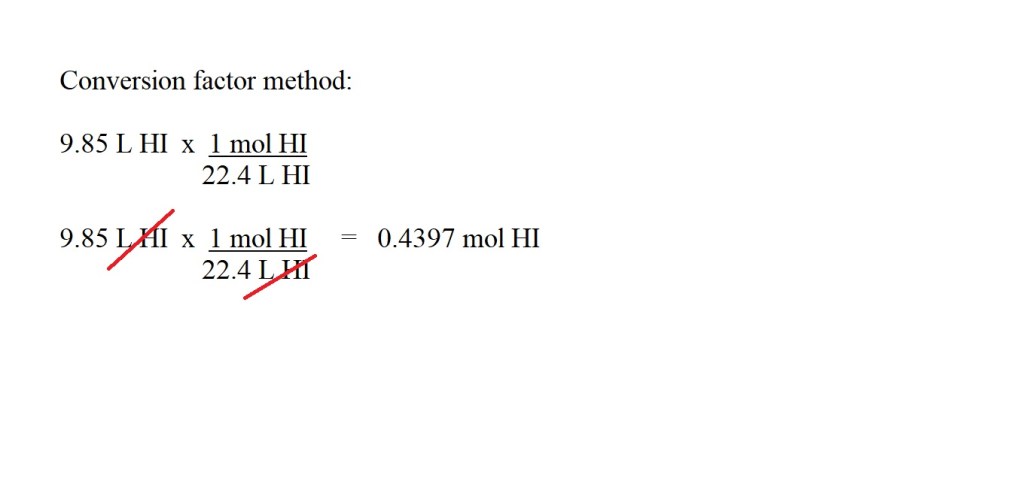
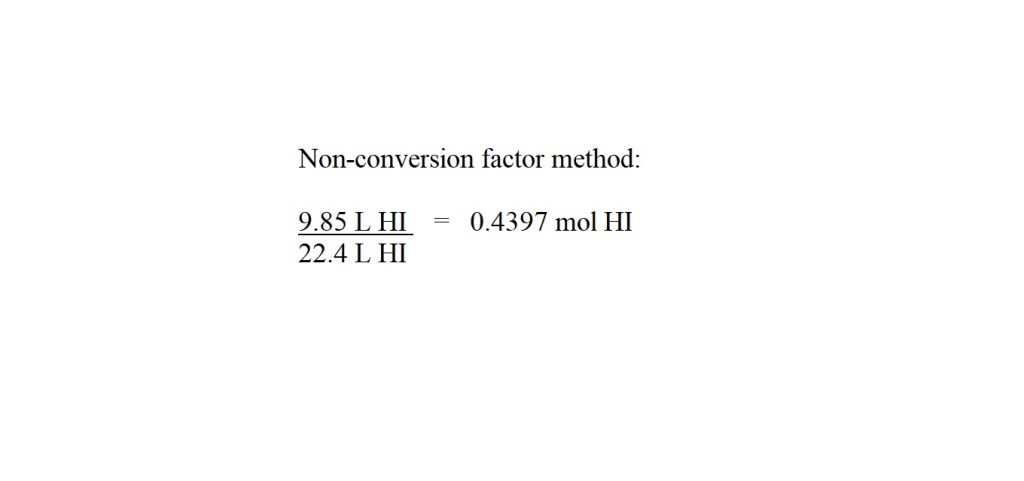
I would advise going for four significant figures for amount in moles if it’s not the final answer to the question.
3) Determine the stoichiometric ratio (mole ratio) for hydrogen gas to hydrogen iodide using the balanced chemical equation.

According to the balanced chemical equation, the mole ratio is 1 mole of hydrogen gas to 2 moles of hydrogen iodide.
4) Use the stoichiometric ratio (mole ratio) to convert from moles of hydrogen iodide to moles of hydrogen gas.

Answer: 0.220 mol H2 (3 significant figures)
Calculating the Mass of a Gas using the STP Method
You’ll need two different sets of equations:
One to convert volume into amount in moles:

A second to convert the amount in moles into mass in grams:

Question: What is the mass in grams of 52.4 dm3 of oxygen at STP?
This is a two-step process:
1) Convert the volume of oxygen into amount in moles.

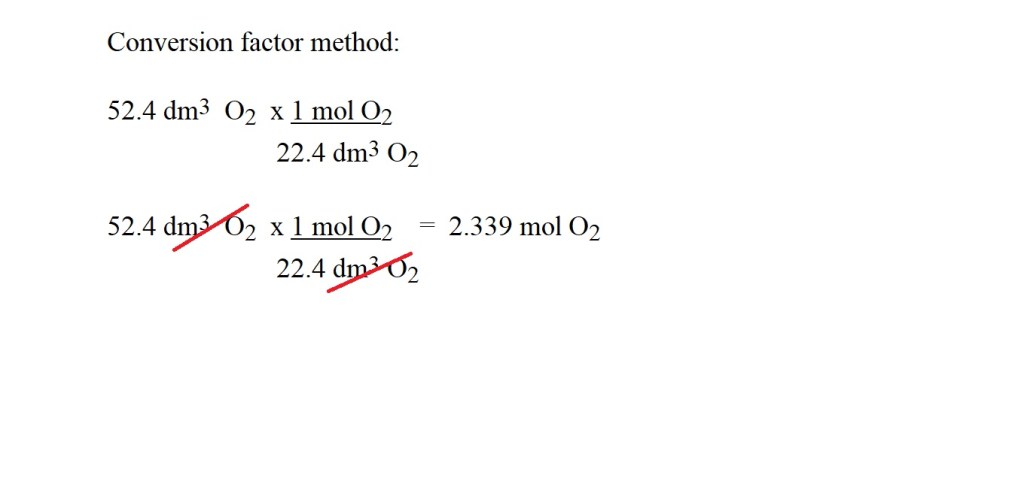

2) Calculate the mass in grams of oxygen using the amount in moles.

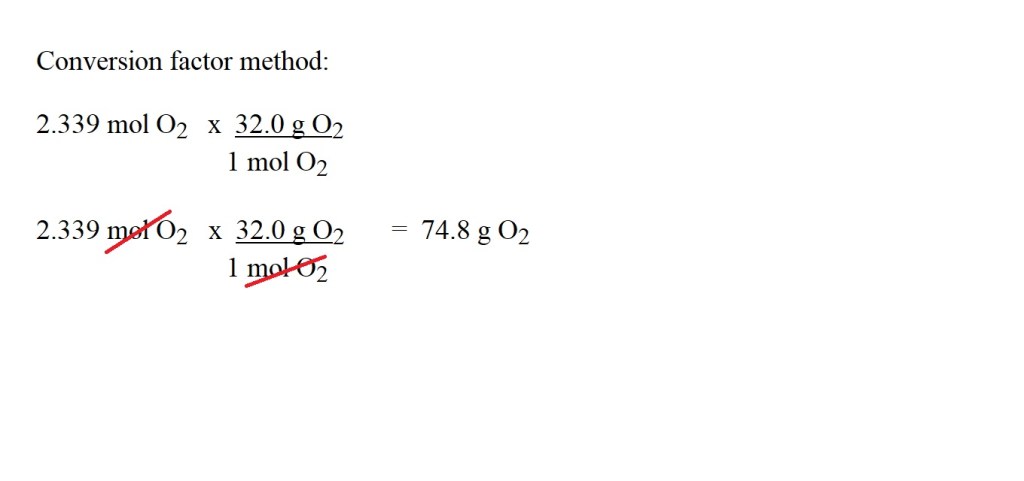

Answer: 74.8 g O2 (to 3 significant figures)
Question: How many grams of propane (C3H8) react with excess oxygen (O2) to produce 54.7 L of carbon dioxide (CO2) and water (H2O) as a by-product at STP?
Note: This is the kind of question that’s designed to test to see if you can apply the correct steps and involve the correct gaseous substances in the calculation. That’s why it’s very important to read the question carefully and not include the wrong substances in the calculation.
We are given the volume of carbon dioxide as a product and we are asked to work out the mass in grams of propane which acts as a reactant. These are the substances involved in the reaction we need to focus on.
Let’s break this down step-by-step and please check each step as you progress through the calculation:
Working:
1) Write out the balanced chemical equation that represents the reaction.
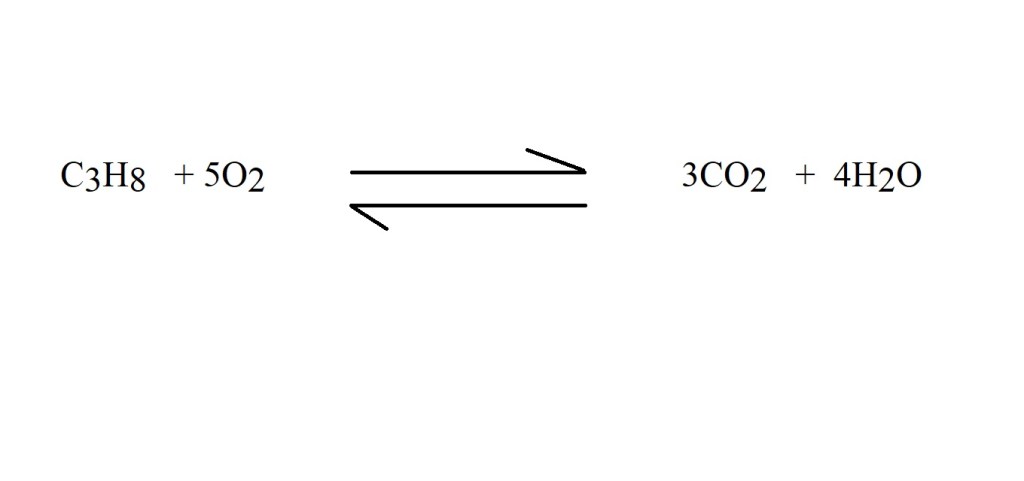
This is the only step in which you have to consider oxygen and water. The rest of the calculation focuses only on propane and carbon dioxide.
2) Convert the volume in litres of carbon dioxide into amount in moles. (Ensure you use the correct units.)

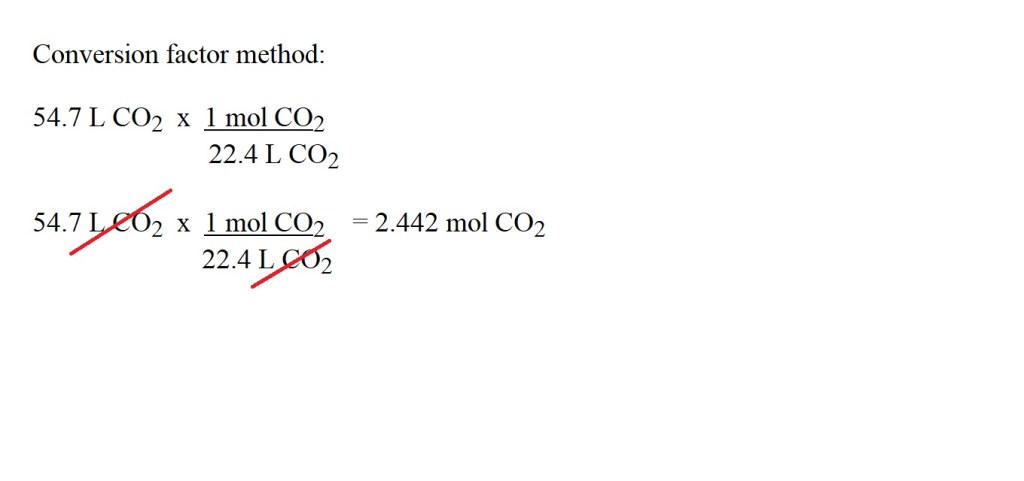

3) Determine the stoichiometric ratio (mole ratio) for propane gas to carbon dioxide using the balanced chemical equation.

According to the balanced chemical equation the mole ratio is 1 mole of propane to 3 moles of carbon dioxide.
4) Use the stoichiometric ratio (mole ratio) to convert from moles of carbon dioxide to moles of propane.
Ensure you write out this step correctly to cancel out the correct units.
Reminder: The numerator in the fraction you are multiplying by is the substance you want to convert to and the denominator is the substance you are converting from.
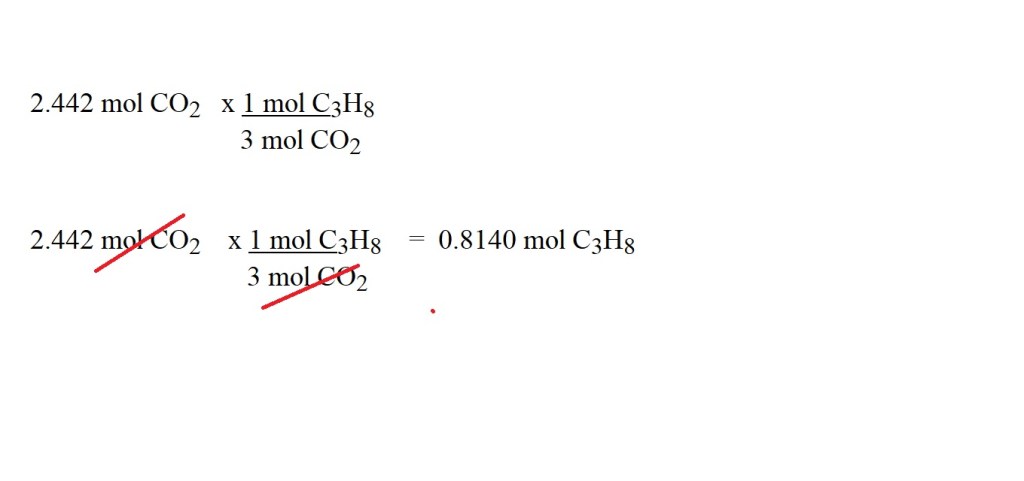
5) Calculate the mass in grams of propane acting as a reactant by using the amount in moles.



Answer: 35.9 g C3H8 (to 3 significant figures).
So, we’ve covered questions in which we’re asked about gases at standard temperature and pressure and we know we’re working with a constant volume for 1 mole of a gas at 22.4 dm3 or 22.4 L. The steps you’ll have to perform varies depending on the question – but as long as the gas is STP the aforementioned constant will apply.
But what about when the temperature and pressure are not in accordance to STP? In the next part of this tutorial we will be examining how to quantify the masses, amounts in moles and volumes of gases at room temperature and pressure (RTP).
You must be logged in to post a comment.