In previous tutorials, we calculated the number of moles of a particular compound present in a reaction, including the number of moles of atoms present using the known number of atoms and Avogadro’s number. However, things can get a bit fiddlier when talking about ions.
Contents:
- Examples of Salt Dissociation
- Mass to Mole Calculations with Ions
- Ion Concentrations to Moles
Examples of Salt Dissociation
When a salt is placed in water, the ionic compound dissociates (separates) into its constituent ions. For example, when sodium chloride (the solute) enters water (the solvent), it dissociates (dissolves) into constituent sodium cations and chloride anions within the resulting solution as demonstrated in the ionic equation below:

s: Solid
aq: aqueous
This process is due to the crystal lattice structure of sodium chloride separating due to the breaking of the electrostatic forces of attraction between the oppositely charged sodium cations and chloride anions. This is followed by the formation of weak electrostatic forces of attraction between slightly (partially) negatively charged oxygen atoms in water molecules and positively charged sodium cations. This is coupled with the formation of weak electrostatic forces of attraction between slightly (partially) positively charged hydrogen atoms in water molecules and negatively charged chloride anions. This leads to the arrangement of water molecules around individual sodium and chloride ions, thereby allowing the latter to be identified as aqueous or hydrated ions.
Reminder: The slightly positively and slightly negatively charged regions of polar molecules is due to the variation in electronegativity of the constituent atoms. This means that atoms such as oxygen are more electronegative and therefore attract shared electrons more than the atom in which it is covalently bonded to such as hydrogen in water; this leads to a relatively more negatively charged region and a relatively more positively charged region.
Let’s now look at a question involving mole calculations with the above example of dissociation.
Important Note: The answers to each question in this tutorial will be given to the same number of significant figures present in the value in the question with the fewest significant figures.
Mass to Mole Calculations with Ions
Question
How many moles of sodium ions are there in 27.0 g of sodium chloride in an aqueous solution?
Let’s take go through this step-by-step.
Step 1:Write out an ionic equation for the dissociation reaction.

Important Note: Ensure you include the charges of the constituent ions, and that the equation is balanced. This example was already balanced, however we will go over another example later on that will require balancing.
Additional Note: Some courses require you to include the states of matter in the ionic equation, others may not. However, it may be considered good practice to get into the habit of including the states of matter in all chemical equations.
Step 2: Calculate the number of moles of sodium chloride in the solution using the provided mass.
Before we can work out the number of moles of sodium, we must first find out the number of moles of sodium chloride in the solution. We need to use the following equation:
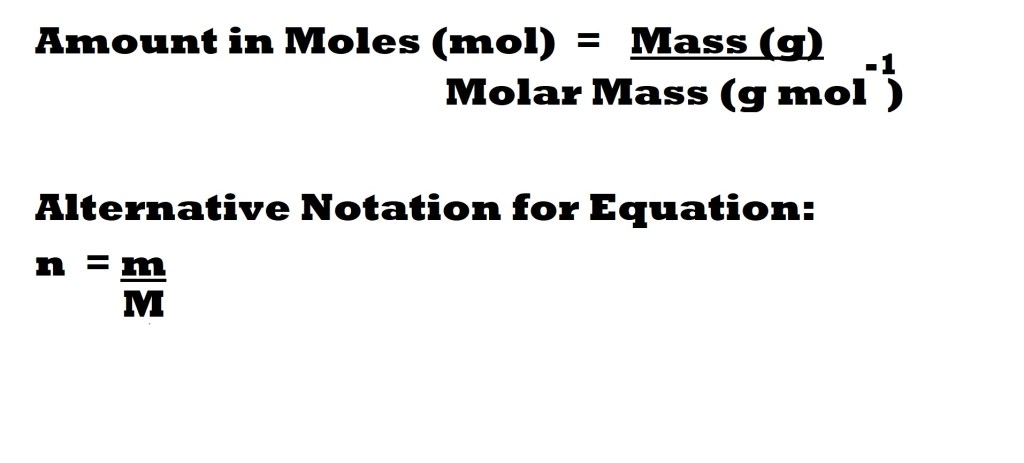
We are given the mass of the sample, so we just need to calculate the molar mass before we can use the equation.
Note: I am going to give the relative atomic masses of elements to four significant figures in this and other calculations in this tutorial. The relative atomic mass can be given in fewer or more significant figures, and it is usually stipulated in the specification for study materials/examinations how many significant figures should be used for your course.
Relative atomic masses (Ar):
Na: 22.99
Cl: 35.45
M = 22.99 + 35.45 = 58.44 g mol-1 NaCl
Let’s now insert those values into the equation and work out the number of moles of sodium chloride.

Step 3: Calculate the number of moles of sodium ions.
(This is the fiddly bit.)
To work out the number of moles of sodium ions, we need to multiply the number of moles of sodium chloride by the number of moles of sodium that are indicated by the mole ratio either in the chemical formula or the balanced ionic equation in step 1. (For simplicity, we are going to stick to the balanced ionic equation method.)
Let’s remind ourselves of the balanced ionic equation for the dissociation of sodium chloride:

Reminder: The absence of any numbers before the chemical formula indicates that 1 mole of the substance is involved.
The equation tells us that for every 1 mole of sodium chloride, there is 1 mole of sodium ions and 1 mole of chloride ions.
This is perhaps a bit clearer if we consider that for every sodium chloride, we need 1 sodium ion and 1 chloride ion and therefore for every 6.02 x 1023 sodium chlorides (1 mole of sodium chloride) we would need 6.02 x 1023 sodium ions (1 mole of sodium ions) and 6.02 x 1023 chloride ions (1 mole of chloride ions).
Therefore, we need to multiply the number of moles of sodium chloride in the solution by the number of moles of sodium for every 1 mole of sodium chloride according to the balanced ionic equation. This will give us the number of moles of sodium ions in the solution.
0.462 mol NaCl x 1.00 mol Na+ = 0.462 mol Na+
Answer: 0.462 mol Na+ are present in a solution containing 27.0g of sodium chloride.
Let’s now look at another – slightly trickier – question.
Question
How many moles of potassium ions are present in 31.0g of potassium sulphate in aqueous solution?
Let’s once again take this step-by-step.
Step 1: Write out an ionic equation for the dissociation reaction.
Reminder: Make sure the equation is balanced and the charges of the ions are indicated.

Step 2: Calculate the number of moles of potassium sulphate present in the solution using the provided mass.
Firstly, we need the molar mass of potassium sulphate.
Relative Atomic Masses (Ar) to 4 significant figures:
K: 39.10
S: 32.07
O: 4 x 16.00 = 64.00
M = 39.10 + 32.07 + 64.00 = 135.2 g mol-1 K2SO4 (to four significant figures)
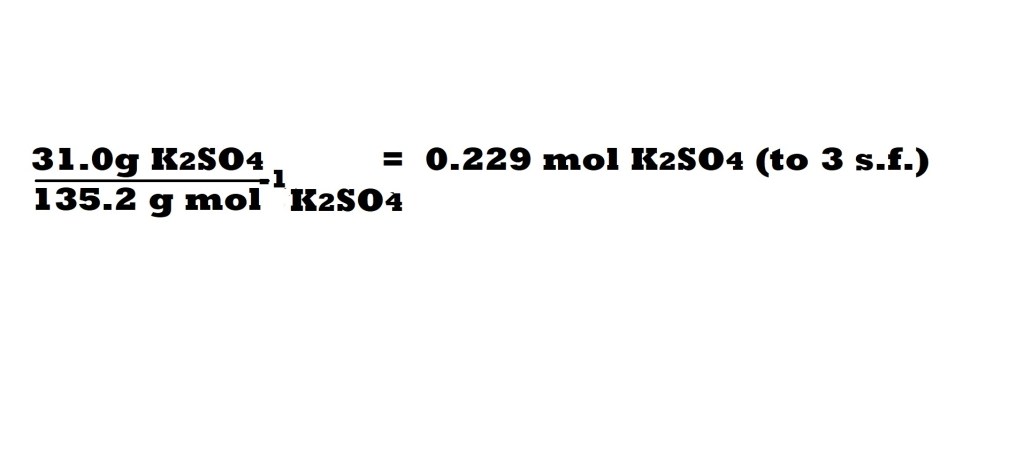
Step 3: Calculate the number of moles of potassium ions present in the solution.
(This is slightly trickier than step 3 in question 1.)
Let’s look at the ionic equation for the reaction again:

This equation tells us that for 1 mole of potassium sulphate that dissociates, 2 moles of potassium cations and 1 mole of sulphate anions are produced.
Therefore, to calculate the number of potassium ions present in the solution, we must multiply the number of moles of potassium sulphate by 2.
0.229 mol K2SO4 x 2.00 mol K+ = 0.458 mol K+
Answer: 0.458 mol K+ are present in a solution containing 31.0 g of potassium sulphate.
Extra Step: Moles of Ions to Number of Ions
Let’s use the answer to the previous question and go a step further to calculate the actual number of potassium ions present in 0.458 mol. To do this we need a familiar number: Avogadro’s number (also known as Avogadro’s constant).
Reminder: Avogadro’s number is 6.02 x 1023 and this represent the number of particles (atoms, ions, molecules or formula units) in 1 mole of a substance.
To calculate the number of ions present we need to use the following equation:
Number of Particles = Amount in Moles (mol) x Avogadro’s Number (NA)
Therefore:
(0.458 mol K+) (6.02 x 1023) = 2.75 x 1023 K+ ions (to 3 significant figures)
Answer: 2.75 x 1023 K+ ions.
Ion Concentrations to Moles
An alternative kind of question involves the concentration of an ionic substance within a solution rather than the initial mass of the sample.
Reminder: Concentration denotes the amount of a substance in moles present per unit of volume of a solution such as cubic decimetres (dm3) or cubic centimetres (cm3).
Note on Other Units for Volume: Volume can also be expressed in litres (L), decilitres (dL) or millilitres (mL). The concentration of a substance can be expressed according to molarity which denotes a concentration with the units of mol L-1. Please check which units are required for your course, however it is usually stated in questions which units should be used.
Important Note: The symbol for molarity is M, the same symbol as for molar mass, therefore it is important to read the rest of a question carefully to be sure of the context.
You may be required to convert between different units for volume such as between cm3 and dm3. We will demonstrate this step in the example questions.
Question (with volume in dm3)
How many moles of chloride ions are present in a 54.0 cm3 solution containing dissociated calcium chloride with a concentration of 2.30 mol dm-3?
Step 1: Write out the ionic equation for the reaction.
Although we are considering different variables to those in previous questions, it’s still recommended to write out the ionic equation to demonstrate the reaction in order to guide the calculation.

Reminder: Make sure the equation is balanced and the charges of the ions are indicated.
Step 2: Convert the volume units.
We have been given the volume of the solution in cubic centimetres (cm3), however the concentration is given in cubic decimetres (dm3), therefore we need to convert the volume of the solution from cubic centimetres to cubic decimetres.
To convert from cm3 to dm3, we must divide the value by 1000 because 1 dm3 is 1000 cm3.
Therefore:

Step 3: Calculate the amount in moles of calcium chloride present in the solution.
This requires the following equation:

Therefore:
2.30 mol dm-3 CaCl2 x 0.0540 dm3 = 0.124 mol CaCl2 ( to three significant figures)
Step 4: Calculate the number of moles of chloride ions present in the solution.
This is the same process as used for calculations involving mass in which we use the mole ratio in the ionic equation to identify what value the amount in moles of CaCl2 should be multiplied by to work out the amount in moles of Cl–.
Let’s look at the ionic equation again:

This demonstrates that for every 1 mole of calcium chloride that is dissociated, 2 moles of chloride ions result in the aqueous solution.
Therefore, to calculate the amount in moles of chloride ions (anions) present in the solution, we must multiply the amount in moles of calcium chloride by 2.
1.24 mol CaCl2 x 2.00 mol Cl– = 2.48 mol Cl– (to three significant figures)
Answer: 2.48 mol Cl– ions (anions) are present in a 54.0 cm3 solution of calcium chloride with a concentration of 2.30 mol dm-3 .
Extra Step: Amount in Moles to Number of Ions
Number of Particles = Amount in Moles (mol) x Avogadro’s Number (NA)
Therefore:
(2.48 mol Cl– ) (6.02 x 1023) = 1.49 x 1024 Cl– ions (to three significant figures)
Question (with volume in L)
How many moles of lithium ions are present in a 12.4 mL solution containing lithium sulphate with a concentration of 3.90 mol L-1?
Reminder: mol L-1 could be expressed in terms of molarity with the symbol M, therefore it could be written as 3.90 M.
Step 1: Write out the ionic equation for the reaction.

Reminder: Make sure the equation is balanced and the charges of the ions are indicated.
Step 2: Convert the volume units.
We are given the volume of the solution in mL, however we need to convert this to L as the concentration given includes the volume in L. We can do this by dividing by 1000.

Step 3: Calculate the amount in moles of lithium sulphate present in the solution.
Amount in Moles (mol) = Concentration (mol L-1) x Volume (L)
3.90 mol L-1 Li2SO4 x 0.0124 L = 0.0484 mol Li2SO4 (to three significant figures)
Step 4: Calculate the number of moles of lithium ions present in the solution.
Let’s look at the ionic equation again:

This indicates that for every 1 mole of lithium sulphate that dissociates, 2 moles of lithium ions (cations) result in the aqueous solution.
Therefore, to calculate the number of moles of lithium ions (cations) present in the solution, we must multiply the amount in moles of lithium sulphate by 2.
0.0484 mol Li2SO4 x 2.00 mol Li2 = 0.0968 mol Li+ (to three significant figures).
Answer: 0.0968 mol Li+ ions (cations) are present in a 12.4 mL solution of lithium sulphate with a concentration of 3.90 mol L-1.
Extra Step: Amount in Moles to Number of Ions
Number of Particles = Amount in Moles (mol) x Avogadro’s Number (NA)
Therefore:
(0.0968 mol Li+) (6.02 x 1023) = 5.83 x 1022 Li+ ions.
Conclusion
In this tutorial we have covered how to calculate the amount in moles of ions present in a solution based on the mass of the original sample and the concentration of the substance.
I hope you found this tutorial helpful and thank you for visiting the site.